GENERATION 2.0
Inspired to build the world’s leading high-science retina pipeline
OUR PIPELINE
Our pipeline is designed to address key limitations of today’s therapies and bring new science to the treatment of retinal diseases.
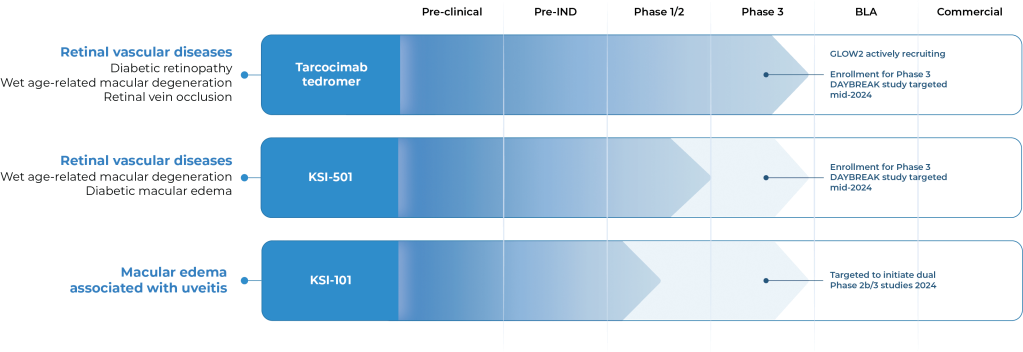
OUR CANDIDATES
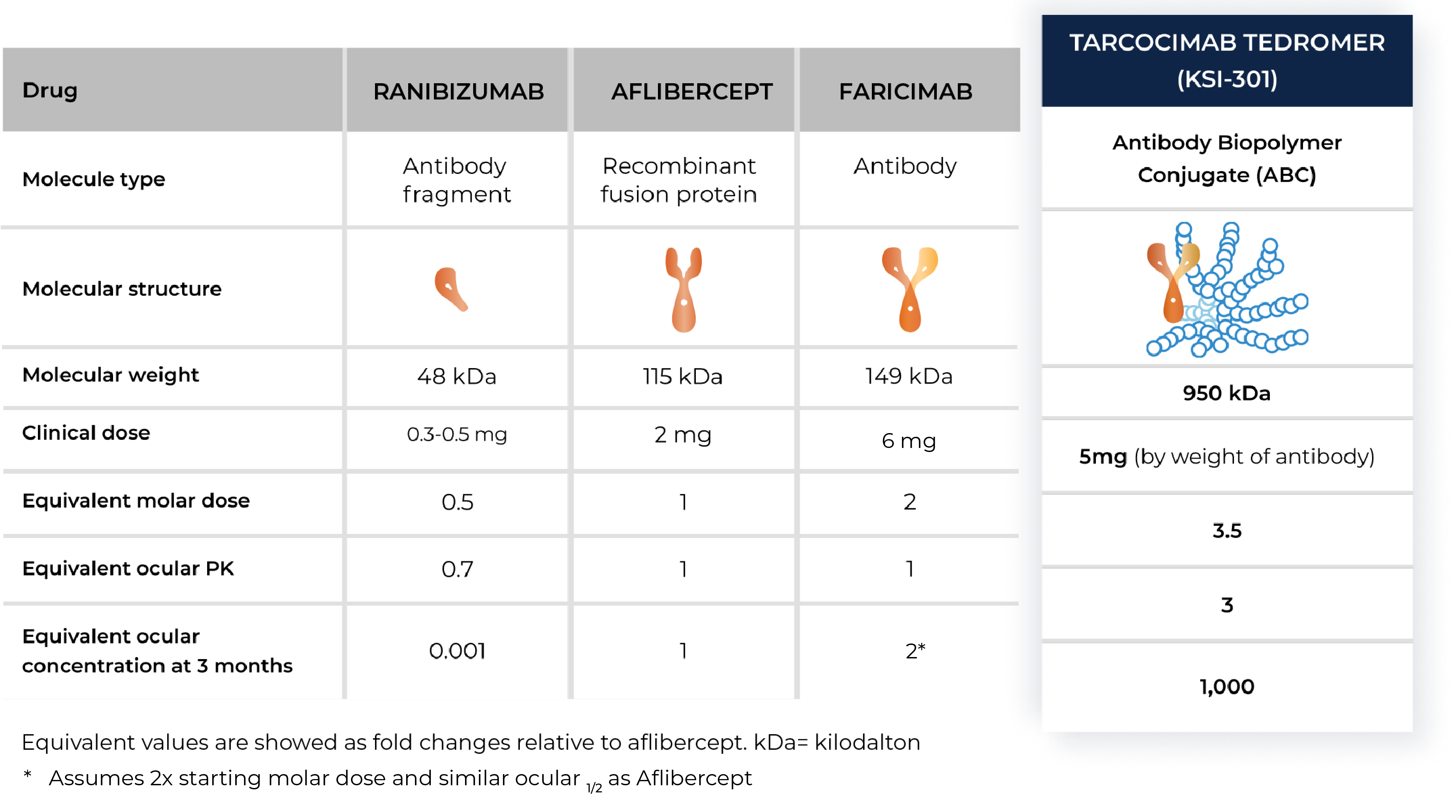
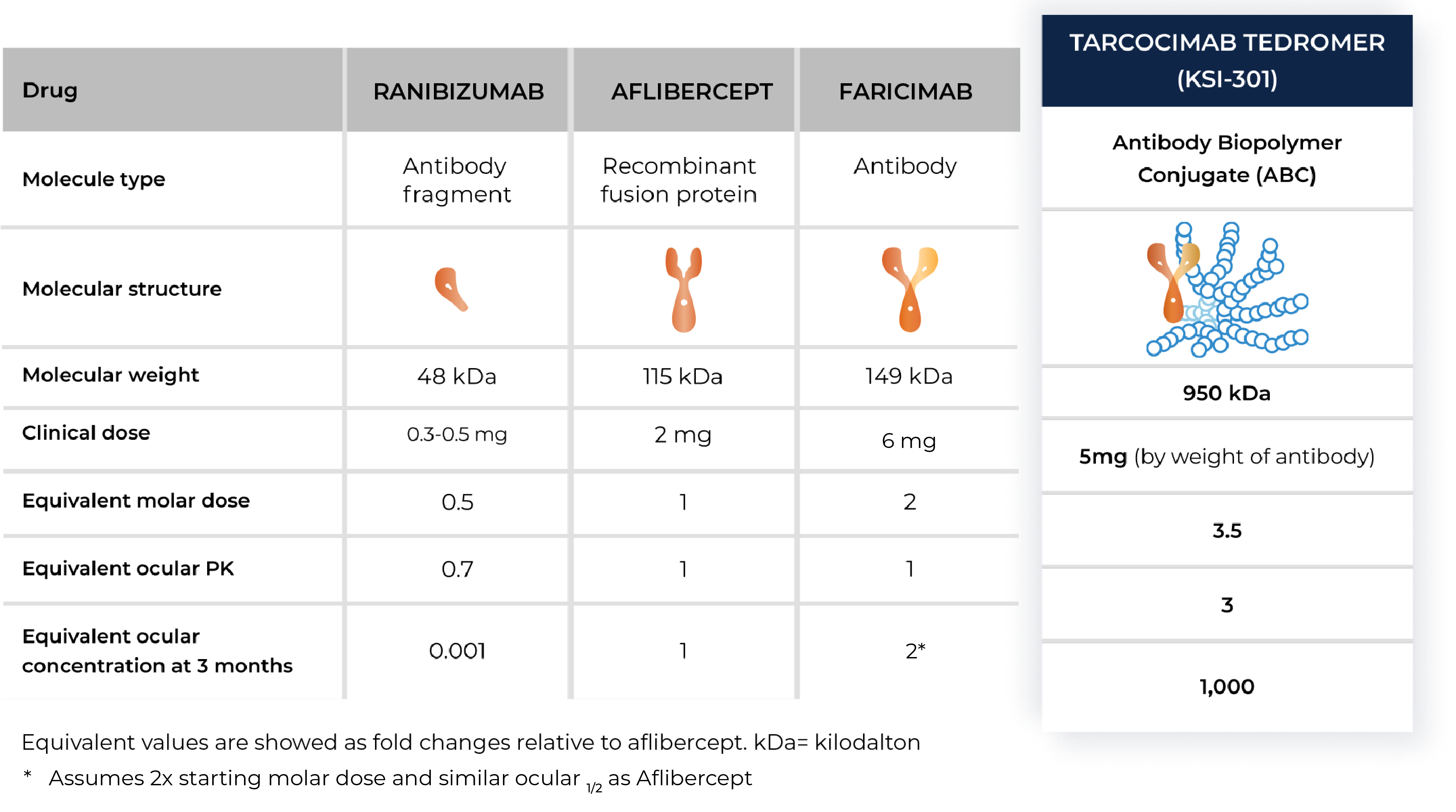
Tarcocimab tedromer
Anti-VEGF
KSI-501
Anti-IL-6, VEGF trap
KSI-101
Anti-IL-6, VEGF trap

Multi-mechanistic medicines
1 Molecule, Many targets
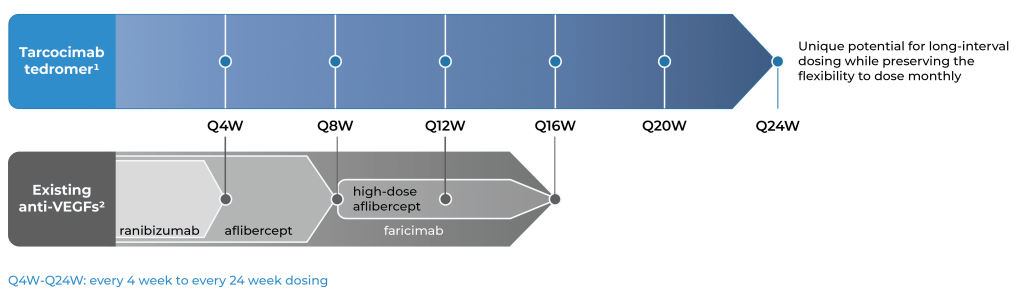
An investigational anti-VEGF biologic designed for long-interval dosing
Tarcocimab is our most advanced program. We have important learnings from six pivotal studies across four major retinal diseases and maintain our conviction that tarcocimab could be an important medicine.
With tarcocimab’s signature durability and safety record, as demonstrated in multiple studies, we believe tarcocimab could be differentiated in the market as a longest-acting biologic based on its ABC Platform design. Our objective is to finish the clinical development program and enable the marketing application. We intend to do this using the go-to-market formulation that we developed in our manufacturing facility, Ursus.
- Across tarcocimab tedromer pivotal studies for diabetic retinopathy, retinal vein occlusion and wet age-related macular degeneration.
- According to the product label for aflibercept, high-dose aflibercept, faricimab and ranibizumab.
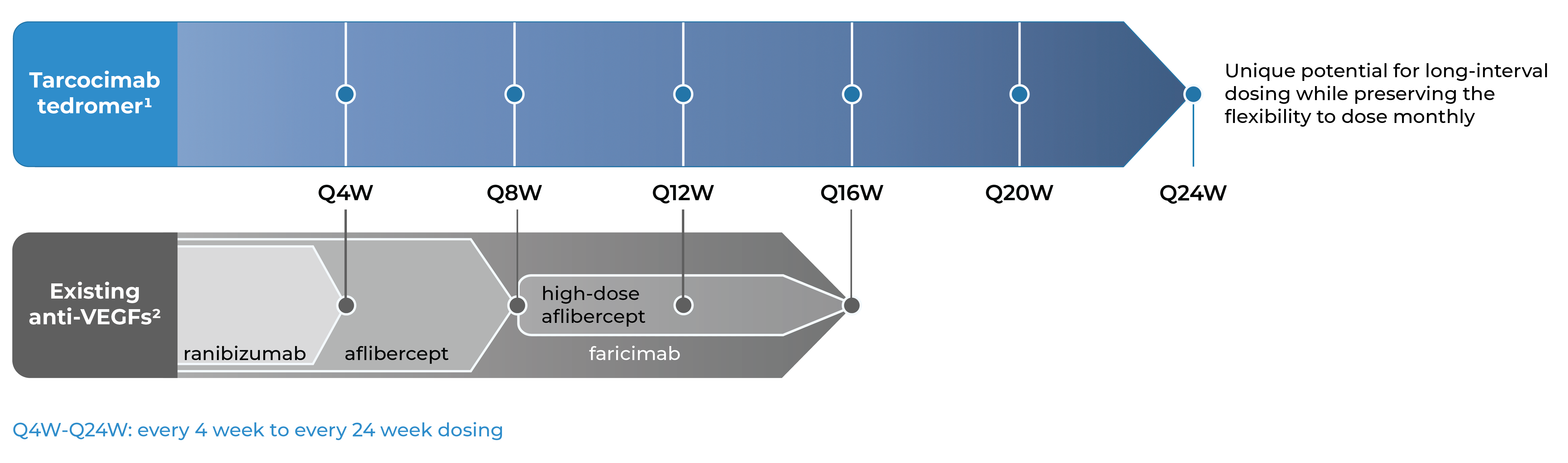
Our Phase 3 studies across high-prevalence retinal diseases
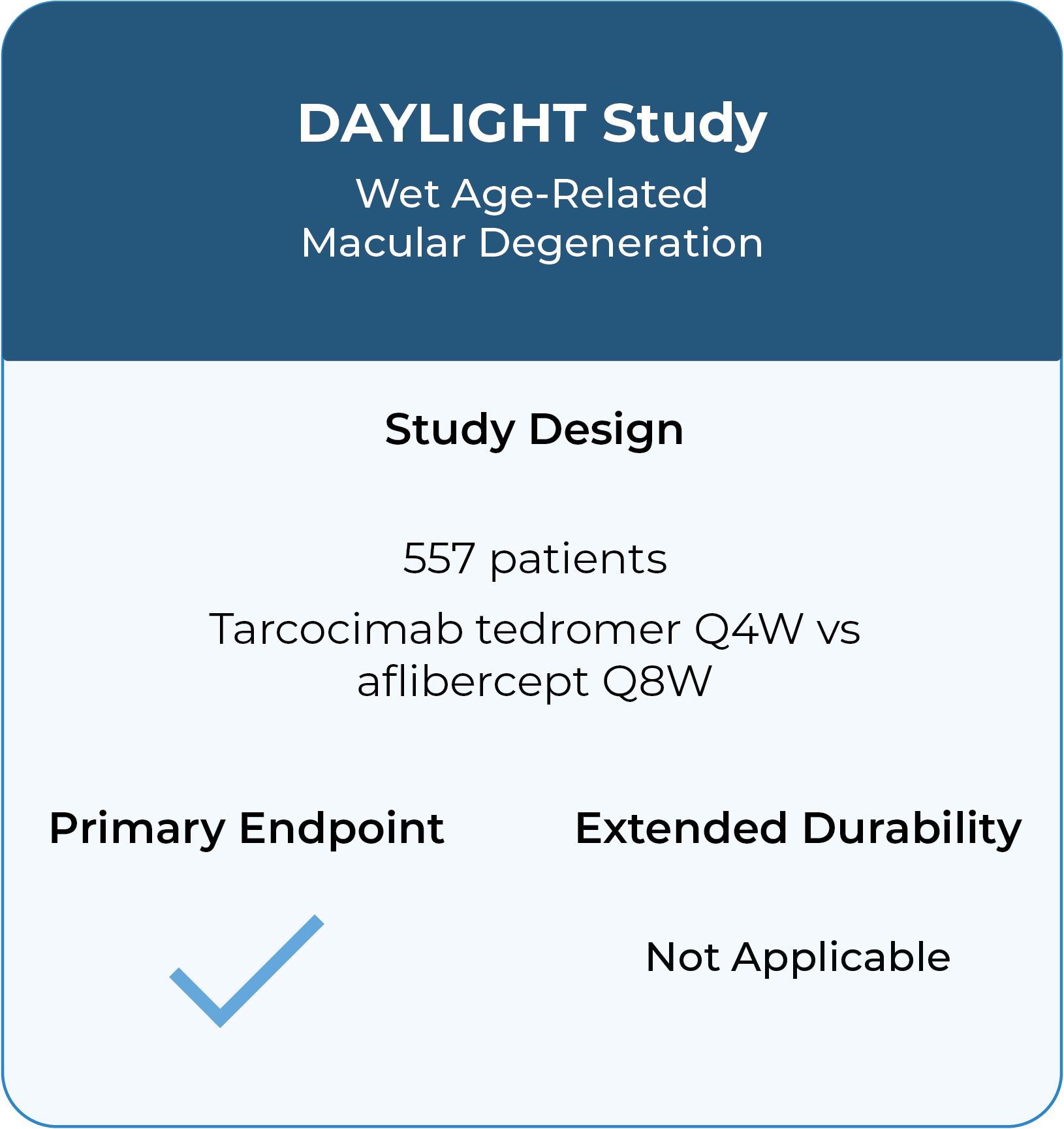
Three Phase 3 studies complete in diabetic retinopathy, retinal vein occlusion and wet age-related macular degeneration with compelling durability demonstrated.
In addition to these studies, tarcocimab was also studied in the Phase 2b/3 DAZZLE study in wet AMD and in the Phase 3 GLEAM and GLIMMER studies in DME. These studies did not meet primary endpoint but did demonstrate strong 5 and 6-month durability in the majority of patients.
Two new Phase 3 studies in process.


Kodiak made adjustments to the tarcocimab product that improve the manufacturability in a prefilled syringe and we believe may also enhance the utility of the product. Both GLOW2 and DAYBREAK will use this go-to-market formulation of tarcocimab.
Our objective is for tarcocimab tedromer to provide class-leading durability while preserving the flexibility to dose monthly for high-need patients
Results from our Phase 3 clinical studies
Tarcocimab demonstrated differentiated durability in the GLOW1 study in diabetic retinopathy and in the BEACON study in retinal vein occlusion.
GLOW1 Phase 3 study in diabetic retinopathy1
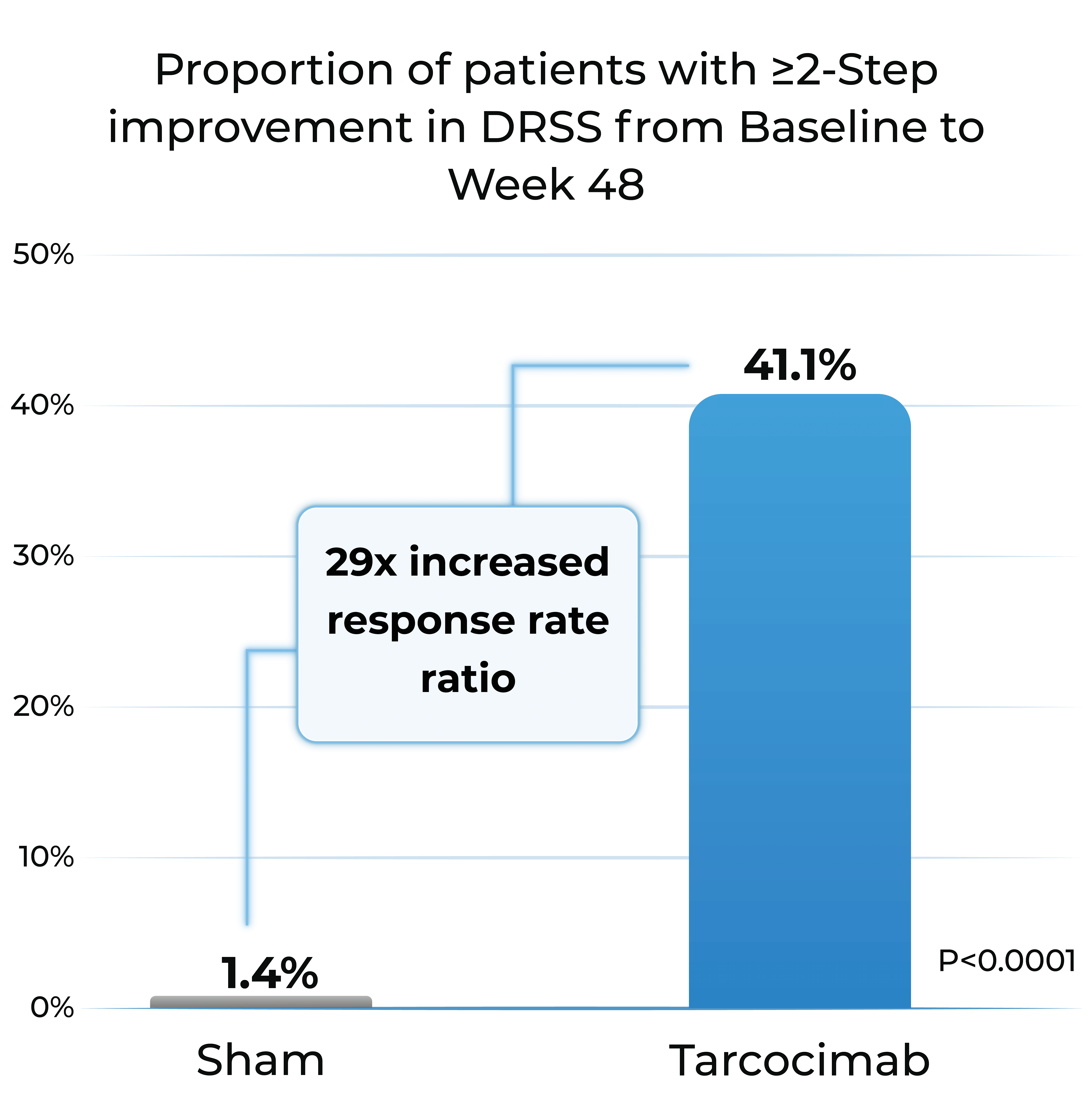
- Patients treated with tarcocimab received only 4 injections in Year 1
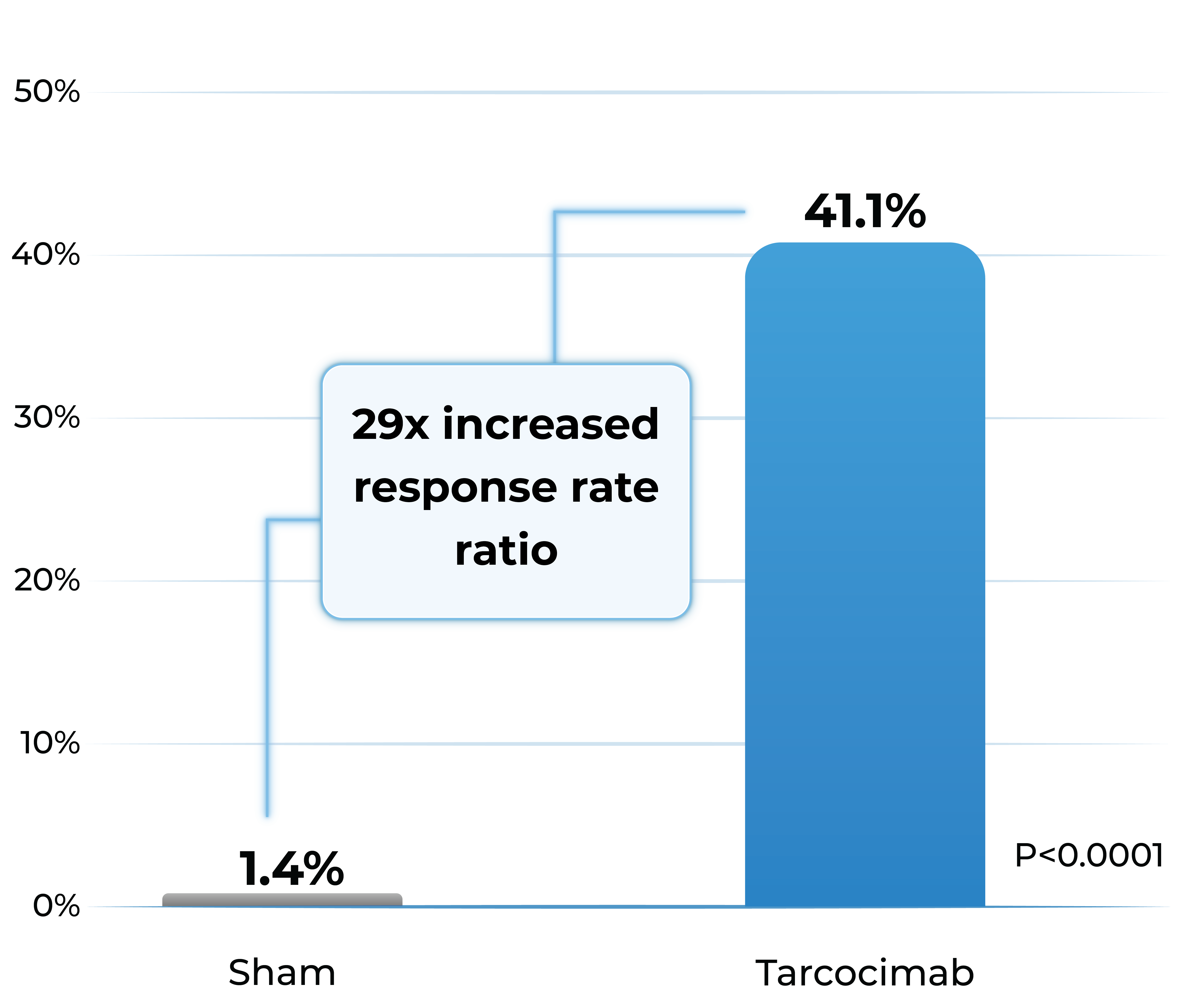
- Tarcocimab demonstrated superiority in ≥2-step and ≥3-step improvement in DRSS
Proportion of patients with ≥2-Step improvement in DRSS from Baseline to Week 48
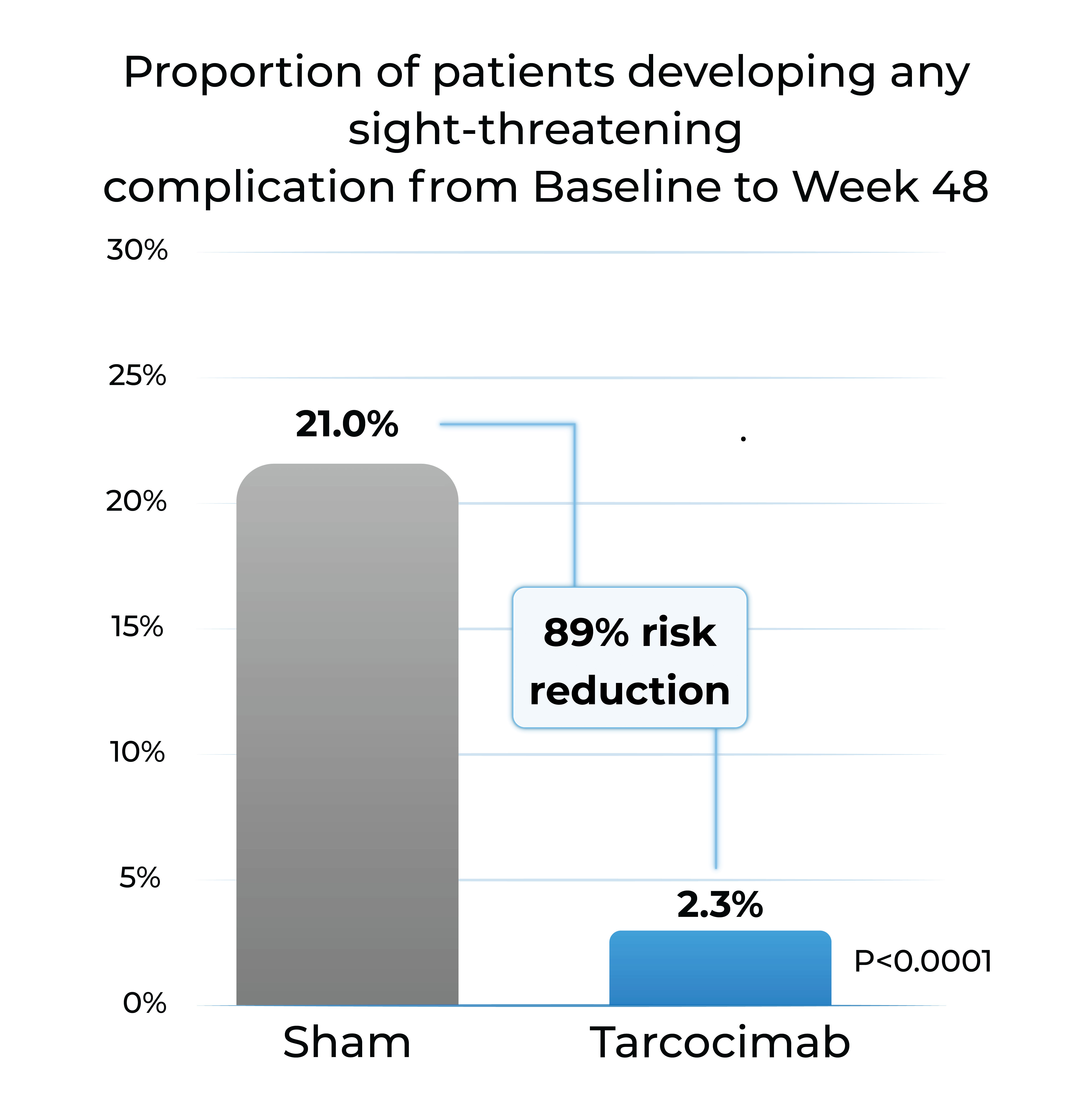
Proportion of patients developing any sight-threatening complication from Baseline to Week 48
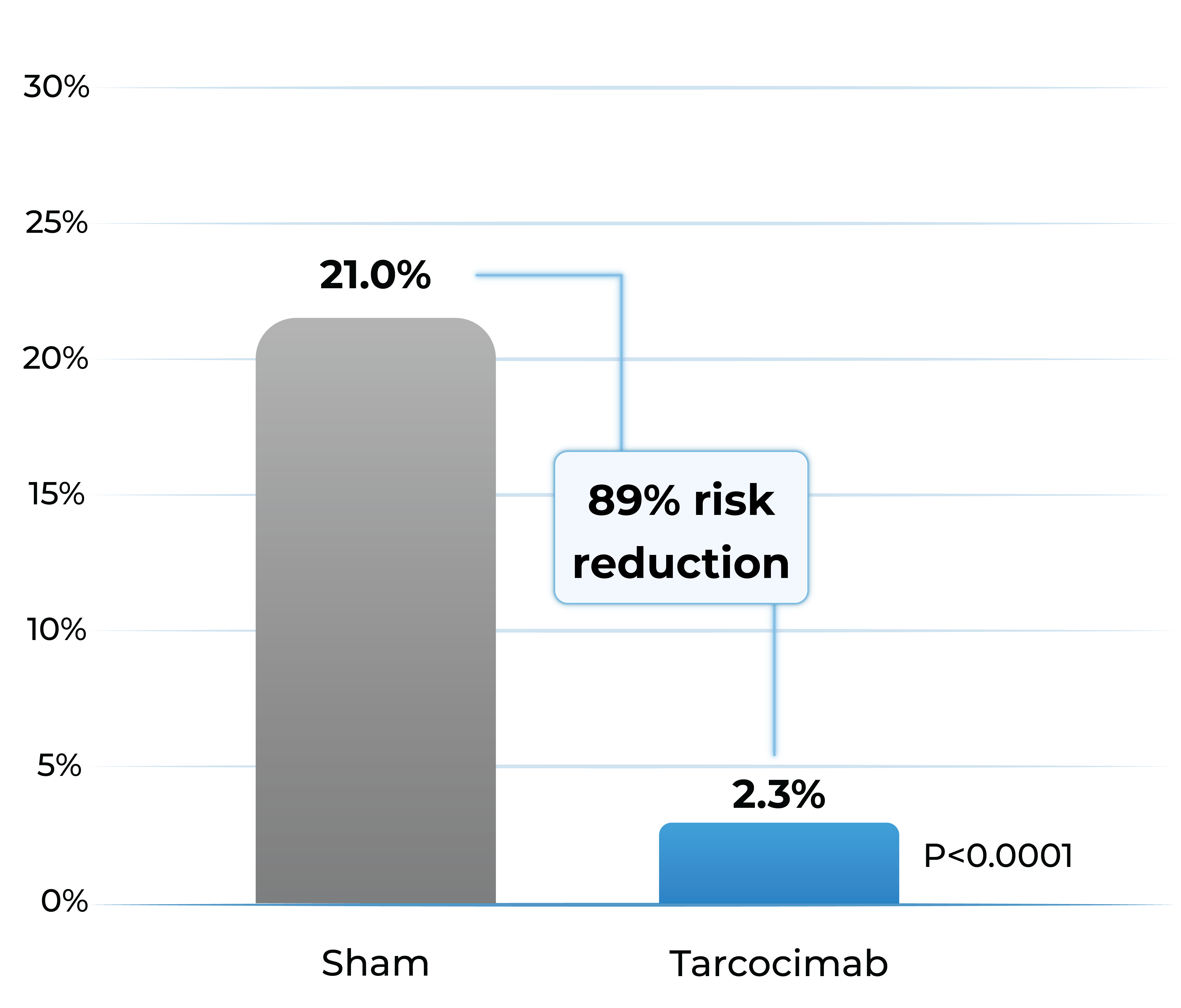
- Tarcocimab reduced the risk of developing a pre-specified sight-threatening complication by ~90%
Any Sight-Threatening Complication
| DME | CST of ≥320 and a 5-letter decrease in BCVA from Day 1; or CST of ≥350 |
| PDR | NVD or NVE, or VH |
| ASNV | ASNV or NVG |
1.The Phase 3 GLOW1 study is a global, multi-center, randomized pivotal study designed to evaluate the efficacy and safety of tarcocimab in patients with treatment-naïve, moderately severe to severe DR. All patients were randomized to receive either tarcocimab every six months after 3 initiating doses or to receive sham injections.
DRSS: diabetic retinopathy severity scale; DME; diabetic macular edema; PDR; proliferative diabetic retinopathy; ASNV: anterior segment neovascularization; CST; central subfield thickness; BCVA; best corrected visual acuity; NVD: neovascularization of the disc; NVE; neovascularization elsewhere; VH: vitreous hemorrhage; NVG; neovascular glaucoma. Weighted percentages are based on weighted average of observed estimates across strata using CMH weights. p-values are based on the difference in response rates.
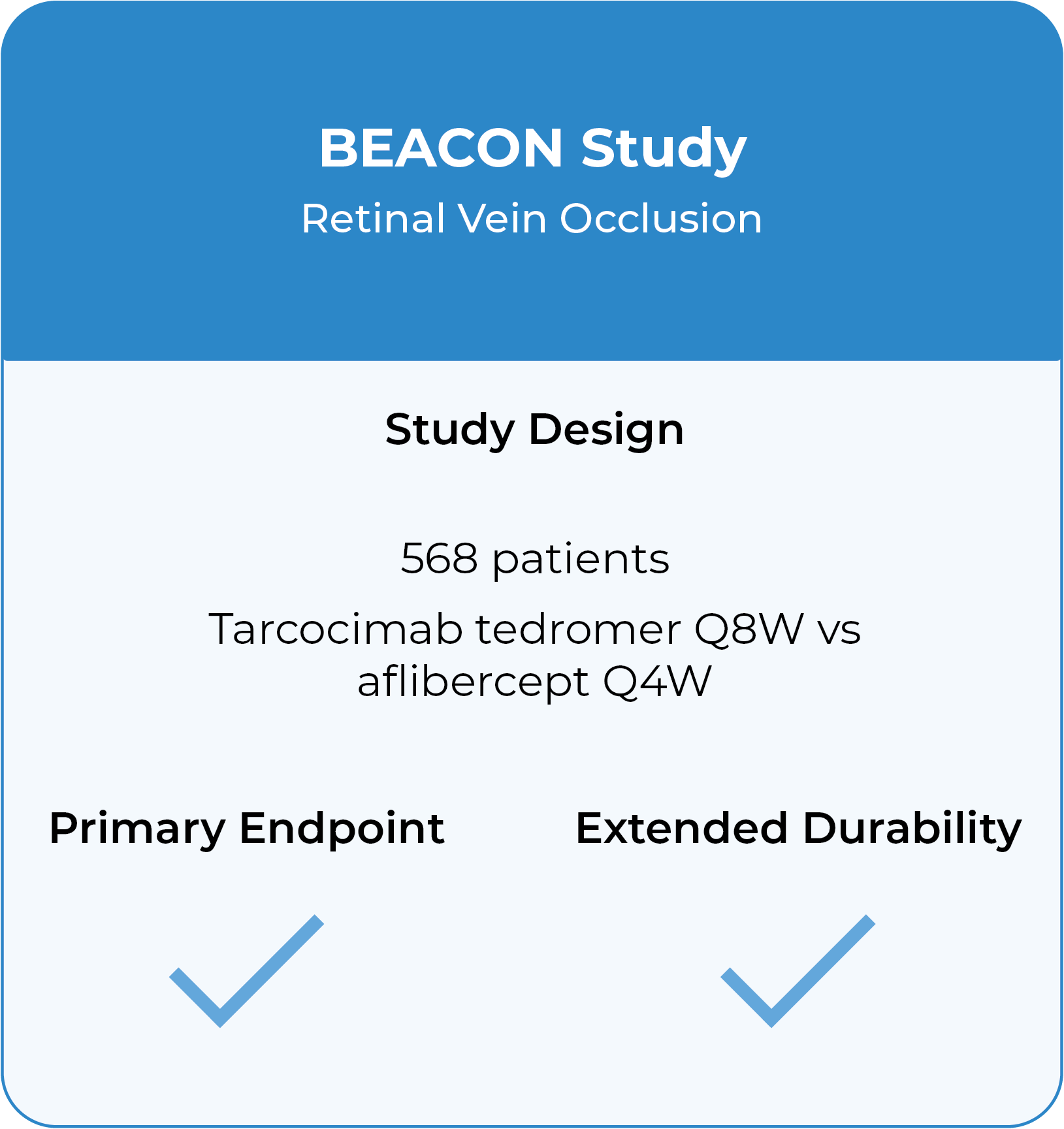
BEACON Phase 3 study in retinal vein occlusion2
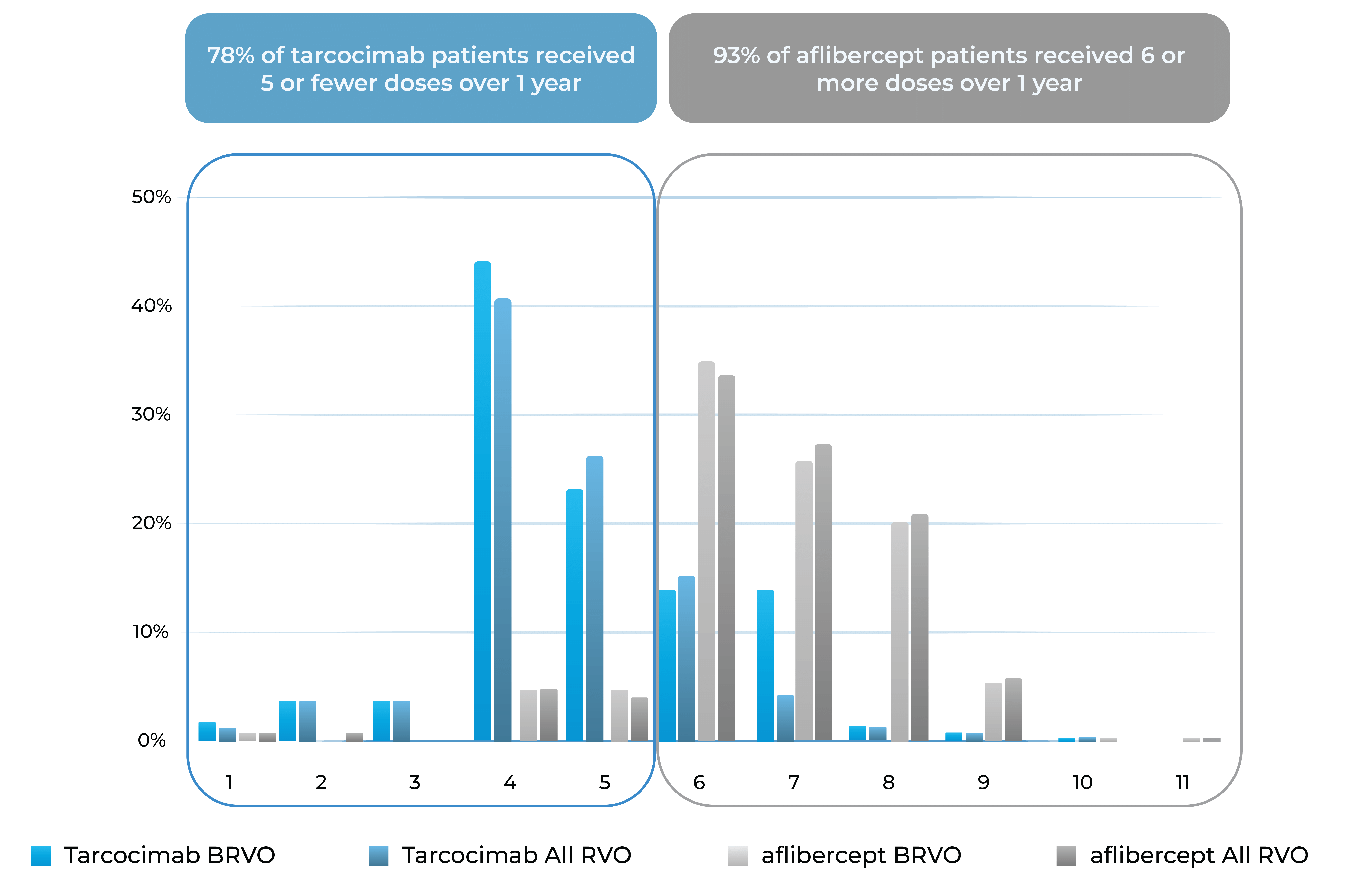
- Tarcocimab Q8W was non-inferior to aflibercept Q4W in all RVO patients at 6 months, thereby doubling the treatment interval
- Approximately half of tarcocimab-treated patients were injection free in the second 6 months of the study
- Despite fewer injections in tarcocimab-treated patients, vision outcomes at Year 1 favored tarcocimab-treated patients achieving an observed mean of 74.6 letters versus 74.3 letters for aflibercept-treated patients
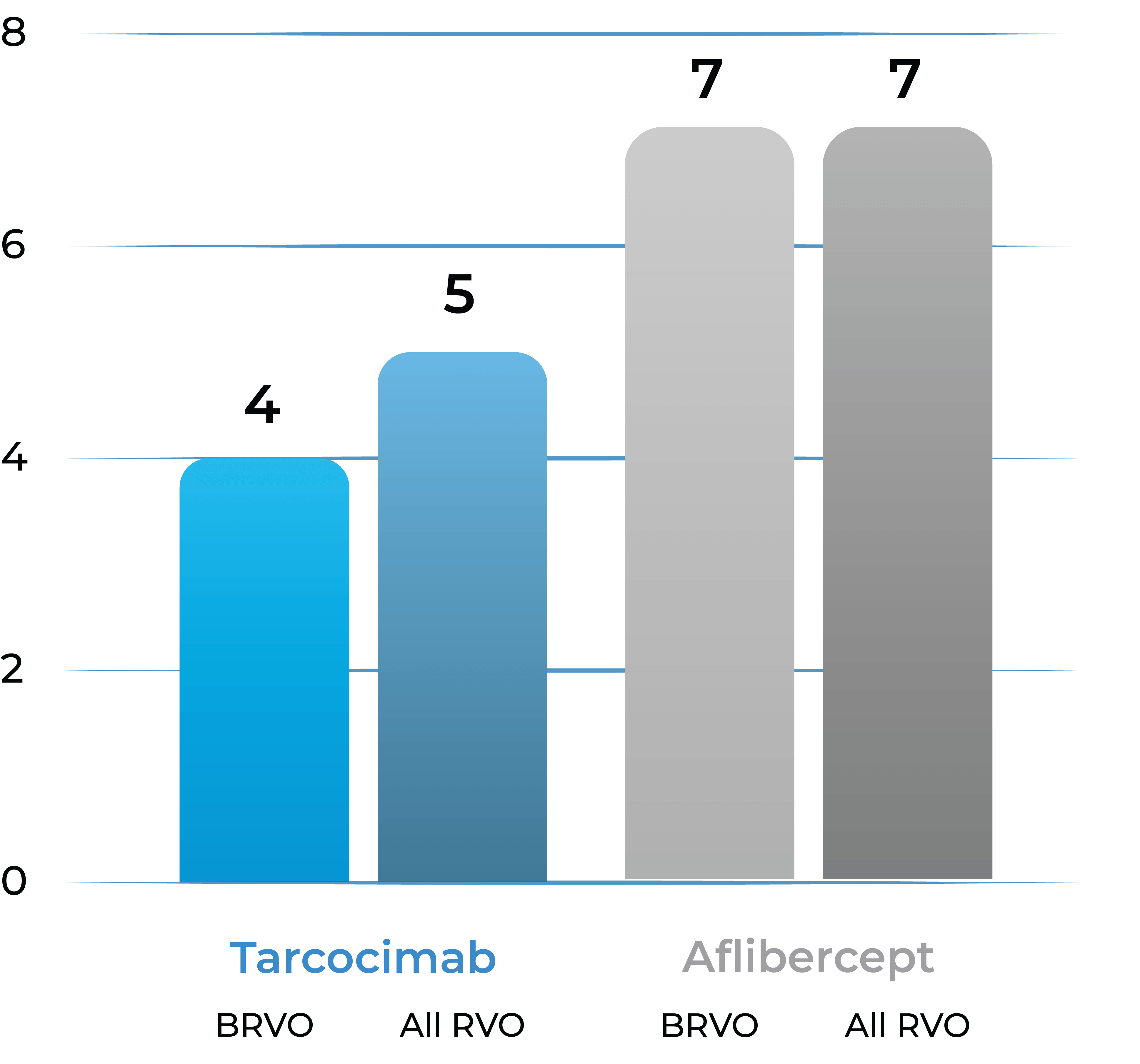
Median number of injections through Week 48
Treatment distribution through Week 48
78% of tarcocimab patients received 5 or fewer doses over 1 year
93% of aflibercept patients received 6 or more doses over 1 year
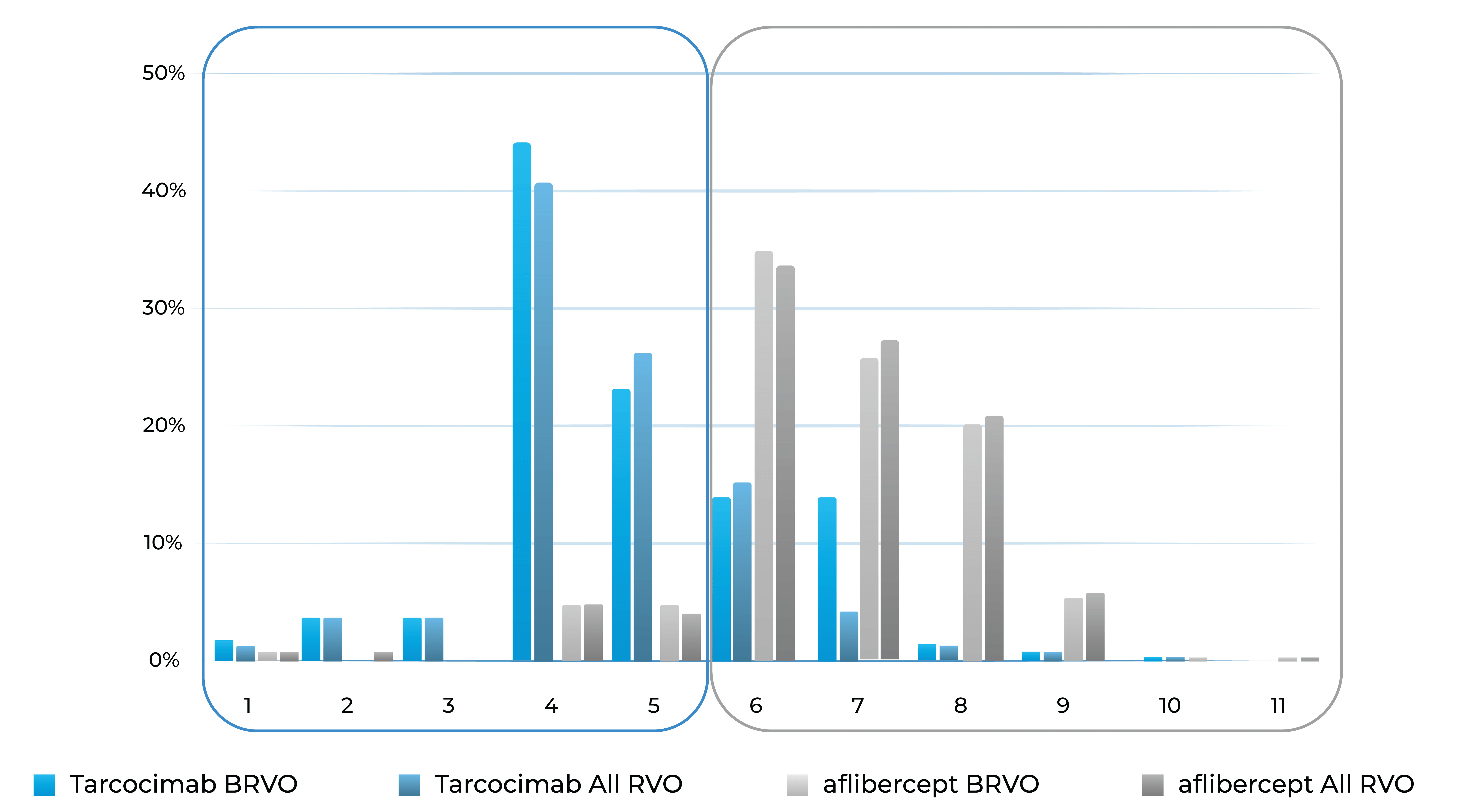
Treatment burden distribution through 48 weeks had minimal overlap, favoring tarcocimab in both BRVO and All RVO patients
2. The Phase 3 BEACON study is a global, multi-center, randomized study designed to evaluate the durability, efficacy and safety of tarcocimab tedromer Q8W vs. aflibercept Q4W in patients with macular edema due to RVO.
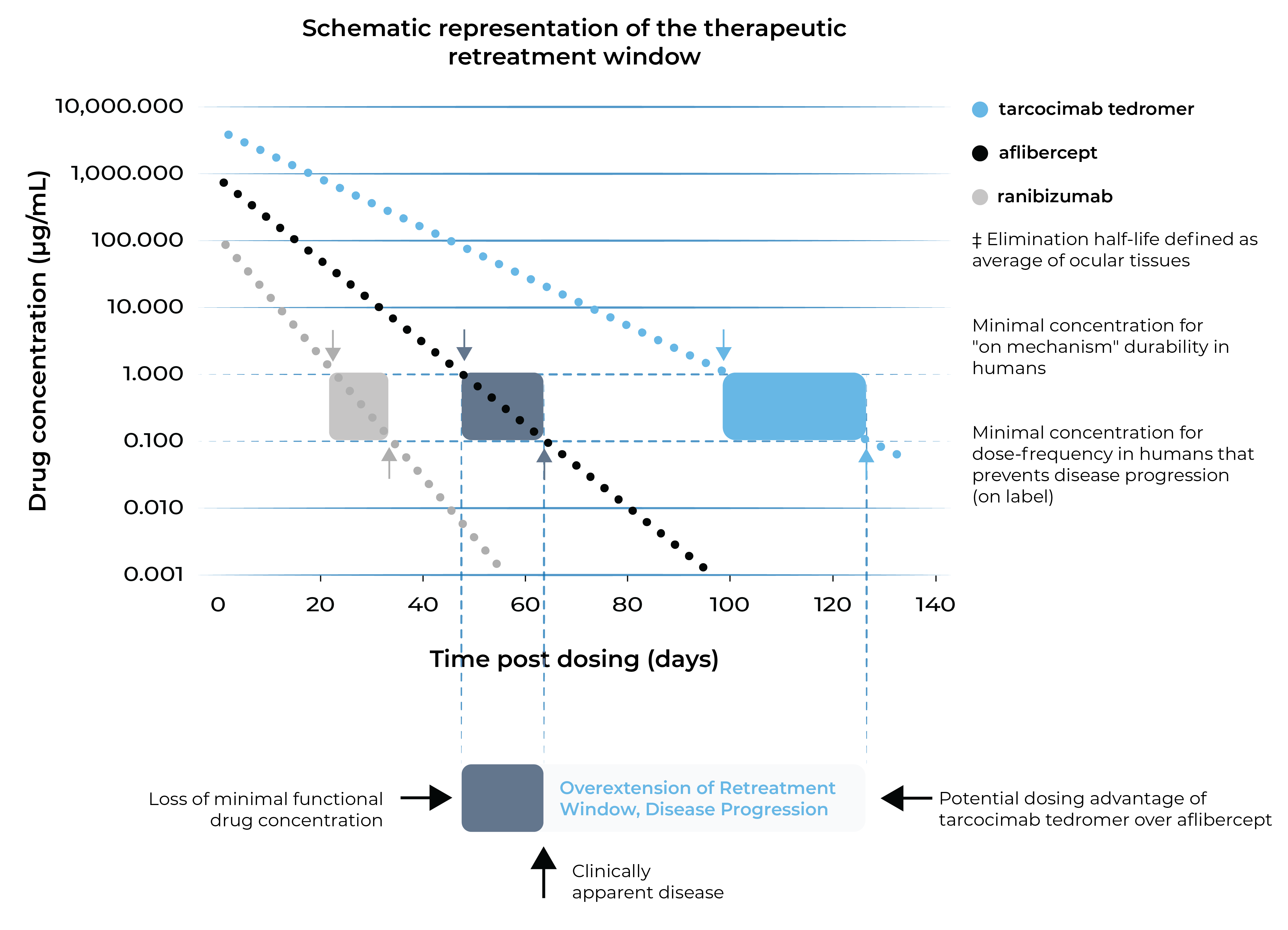
Tarcocimab tedromer and the ABC Platform are purposefully designed for increased durability
Tarcocimab tedromer aims to maintain therapeutic activity for longer.
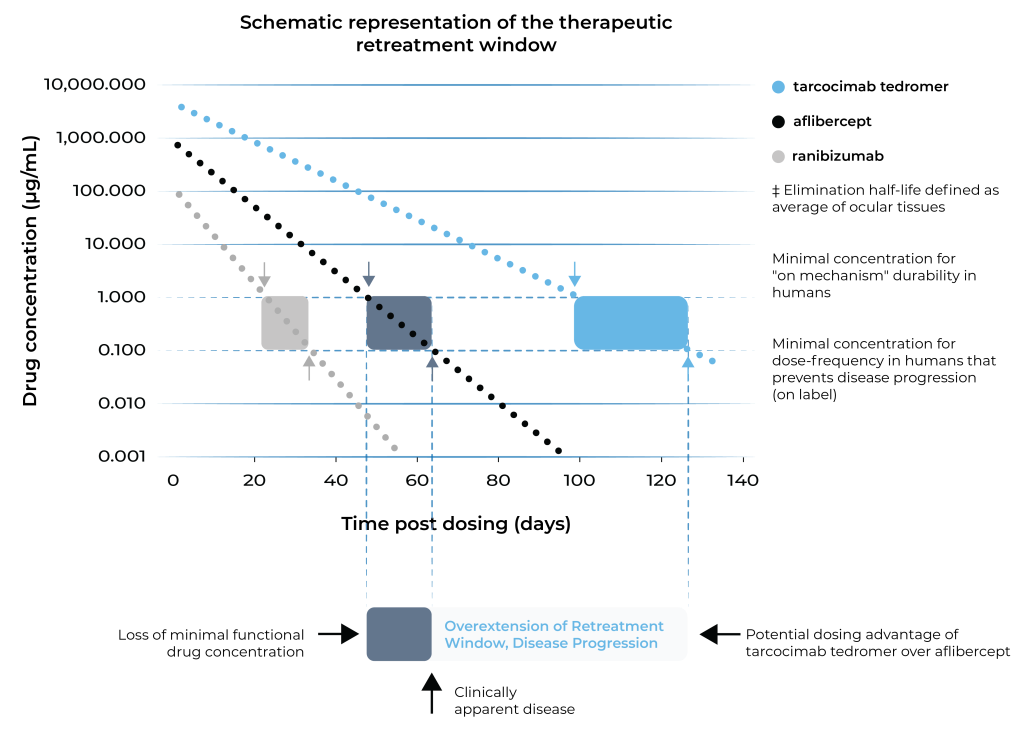
First-in-class
An investigational dual inhibitor biologic designed to address both vascular permeability and inflammation for high-prevalence retinal vascular diseases
Inflammation has been shown to play a significant role in high-prevalence retinal vascular diseases. However, no treatments exist that concurrently address vascular permeability and inflammation.
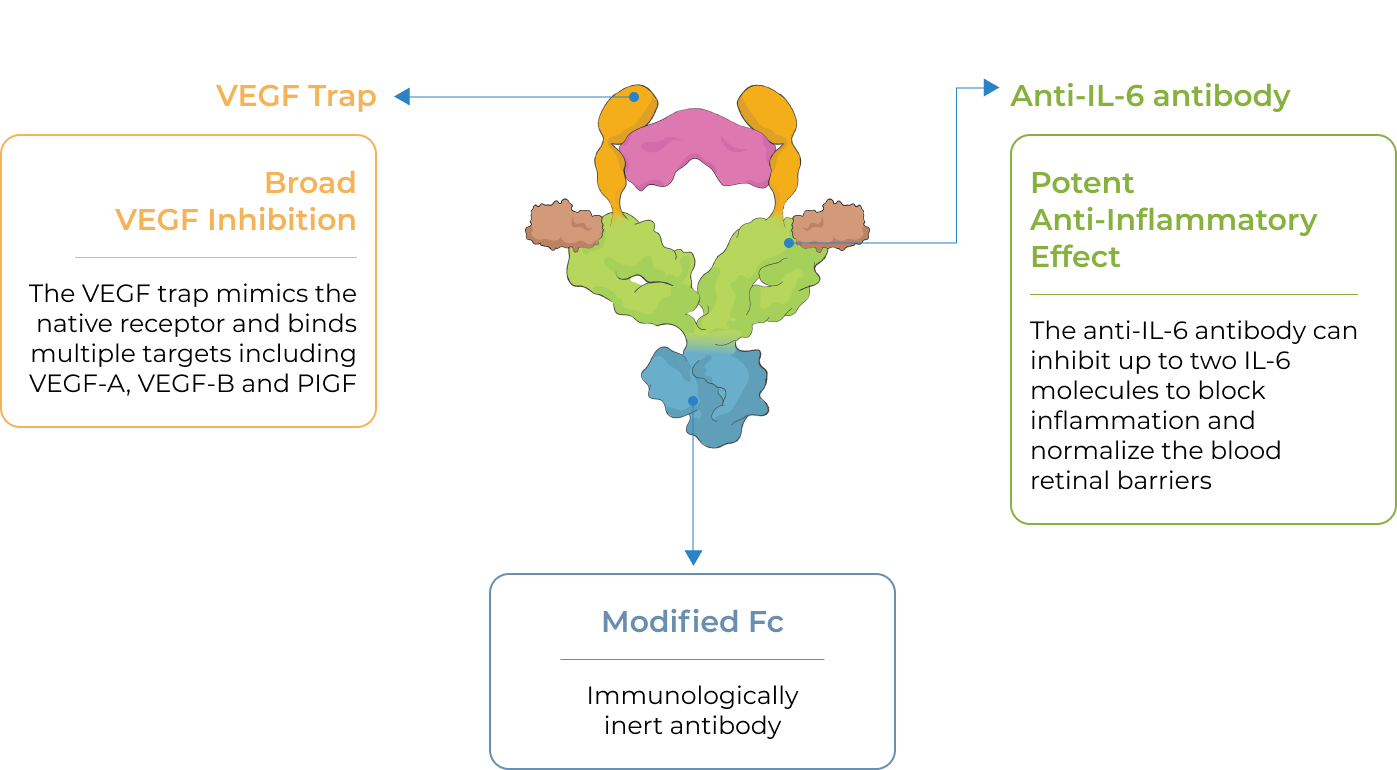
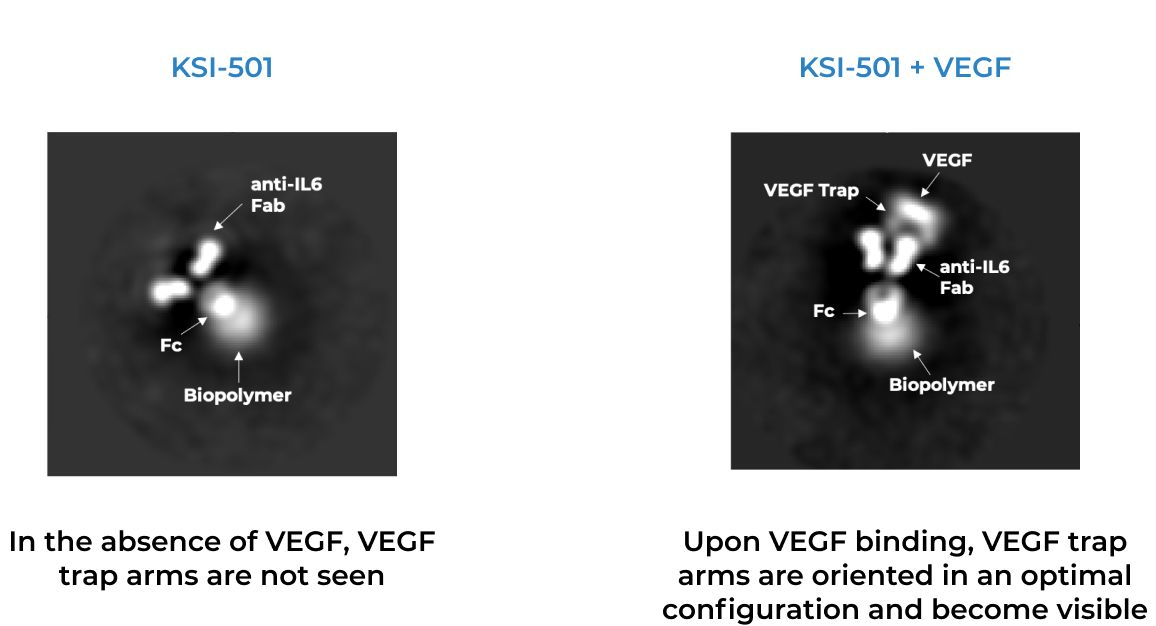
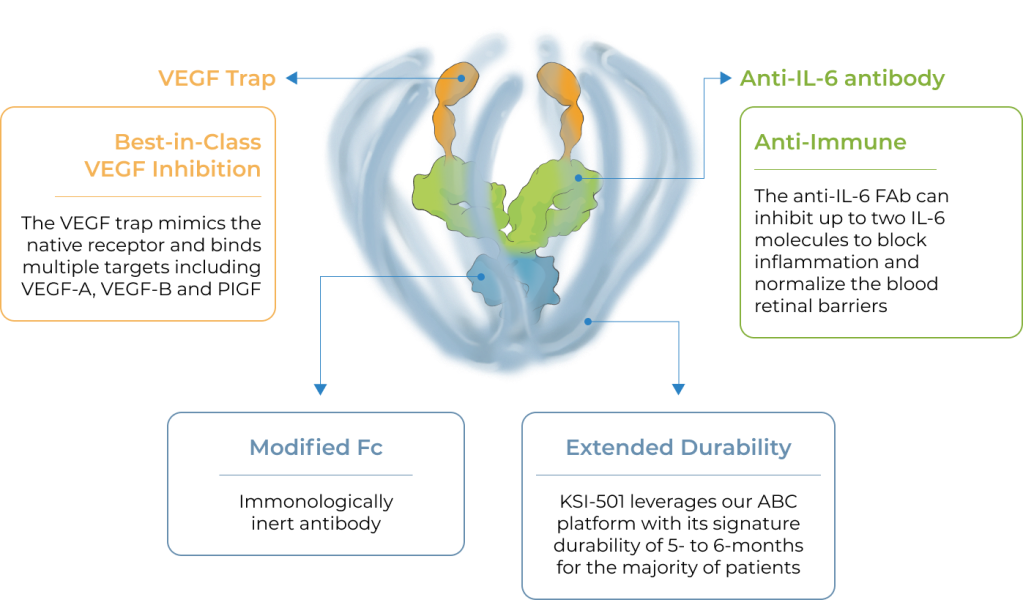
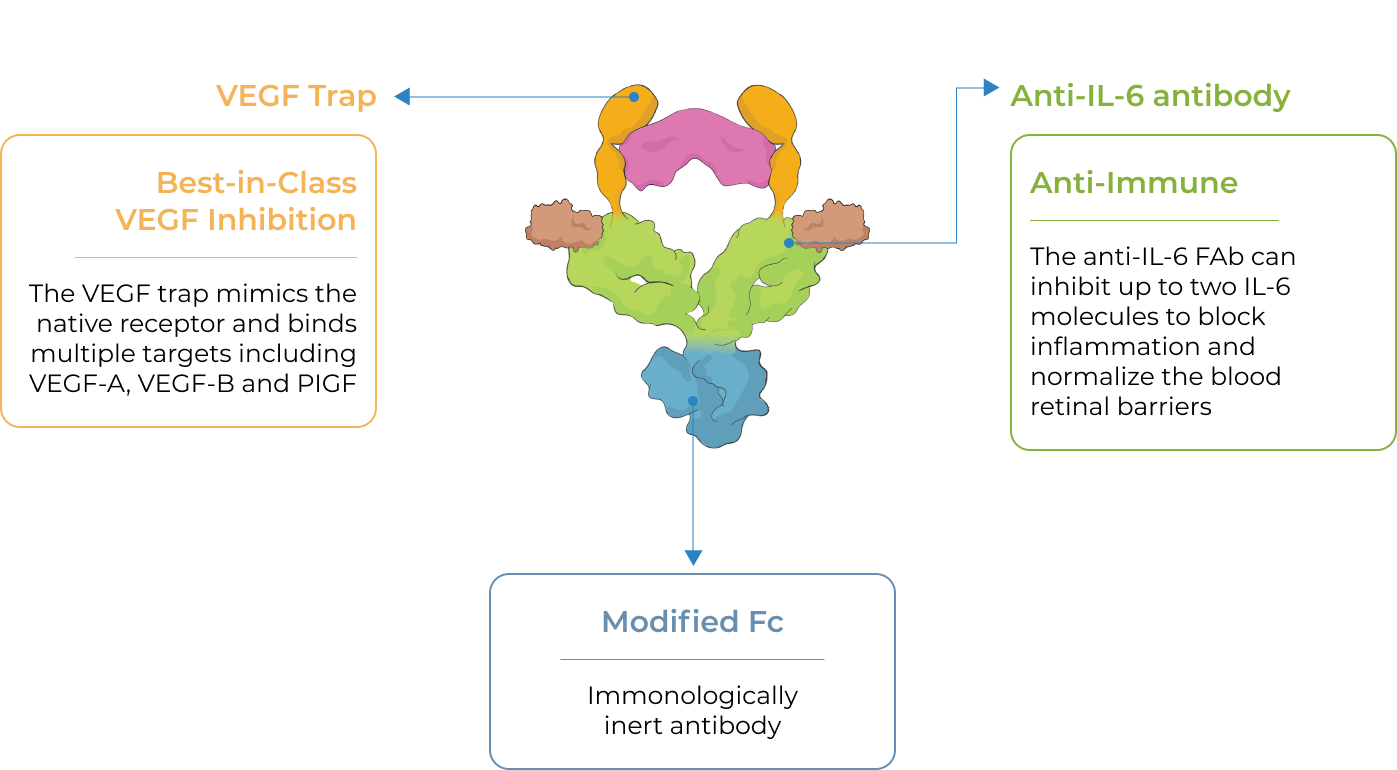
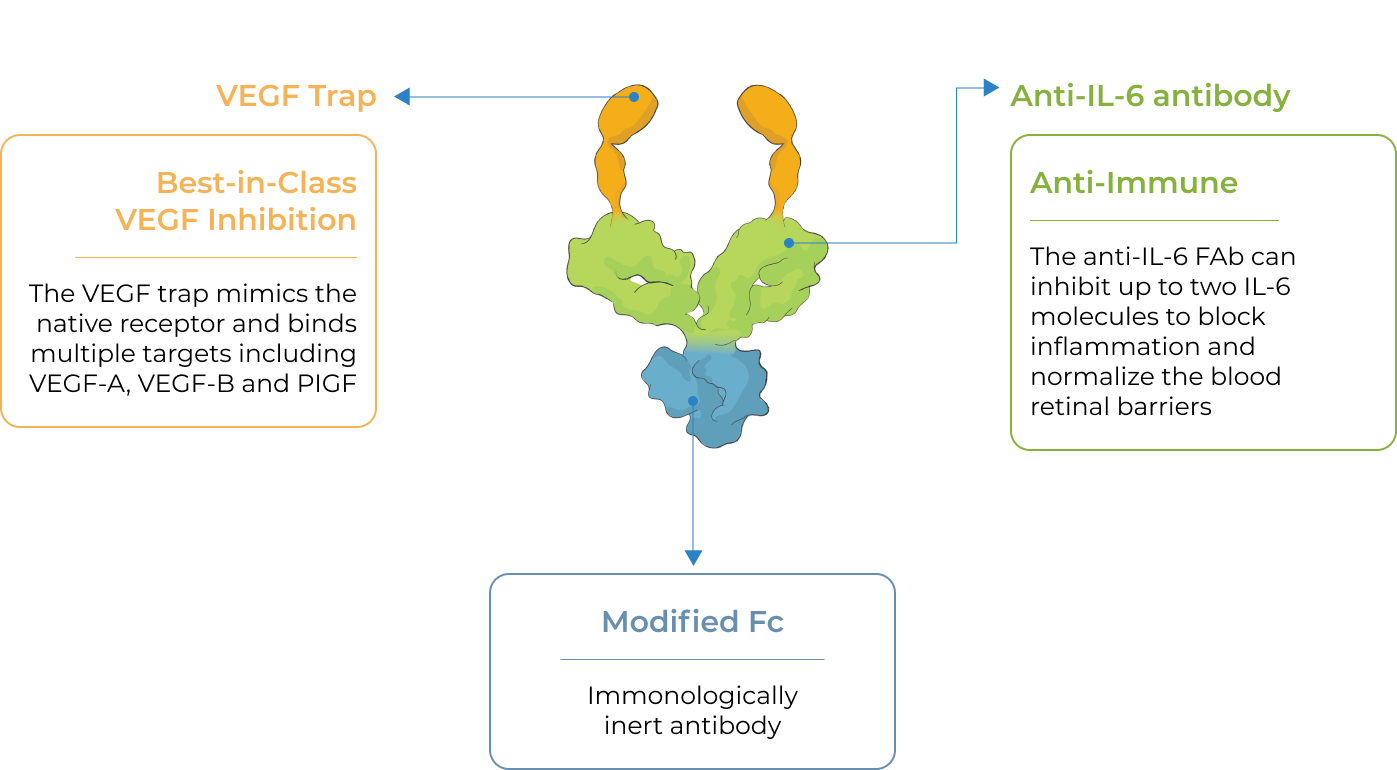
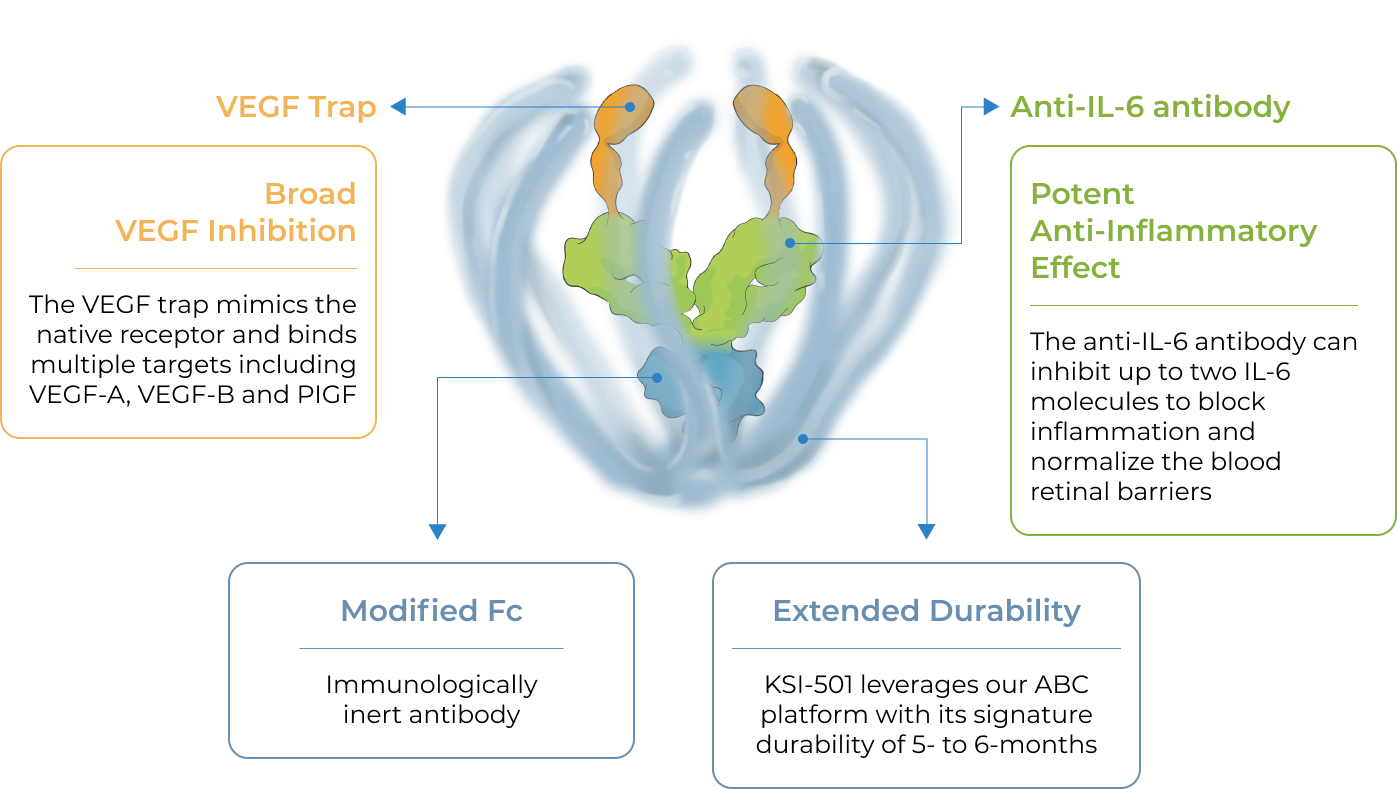
KSI-501, our second investigational medicine built on our Antibody Biopolymer Conjugate (ABC) platform, is designed to inhibit VEGF and IL-6, a pro-inflammatory cytokine and immune growth factor, combining two powerful mechanisms of action to address retinal vascular disease and the underlying inflammatory cascade.
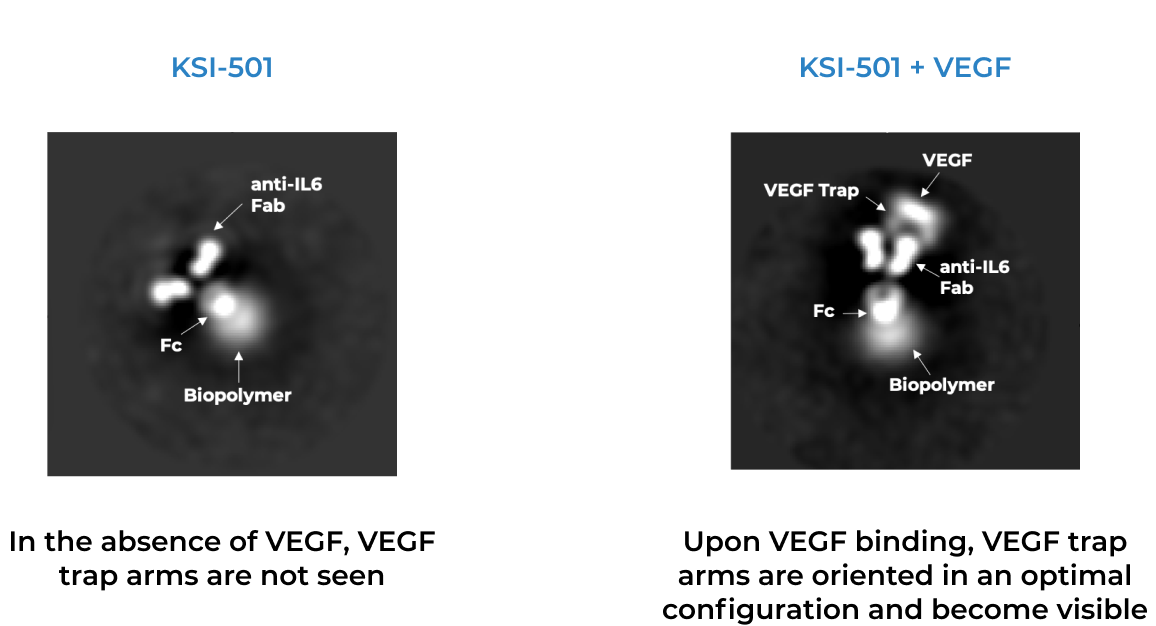
KSI-501 is designed for highly efficient binding to both IL-6 and VEGF
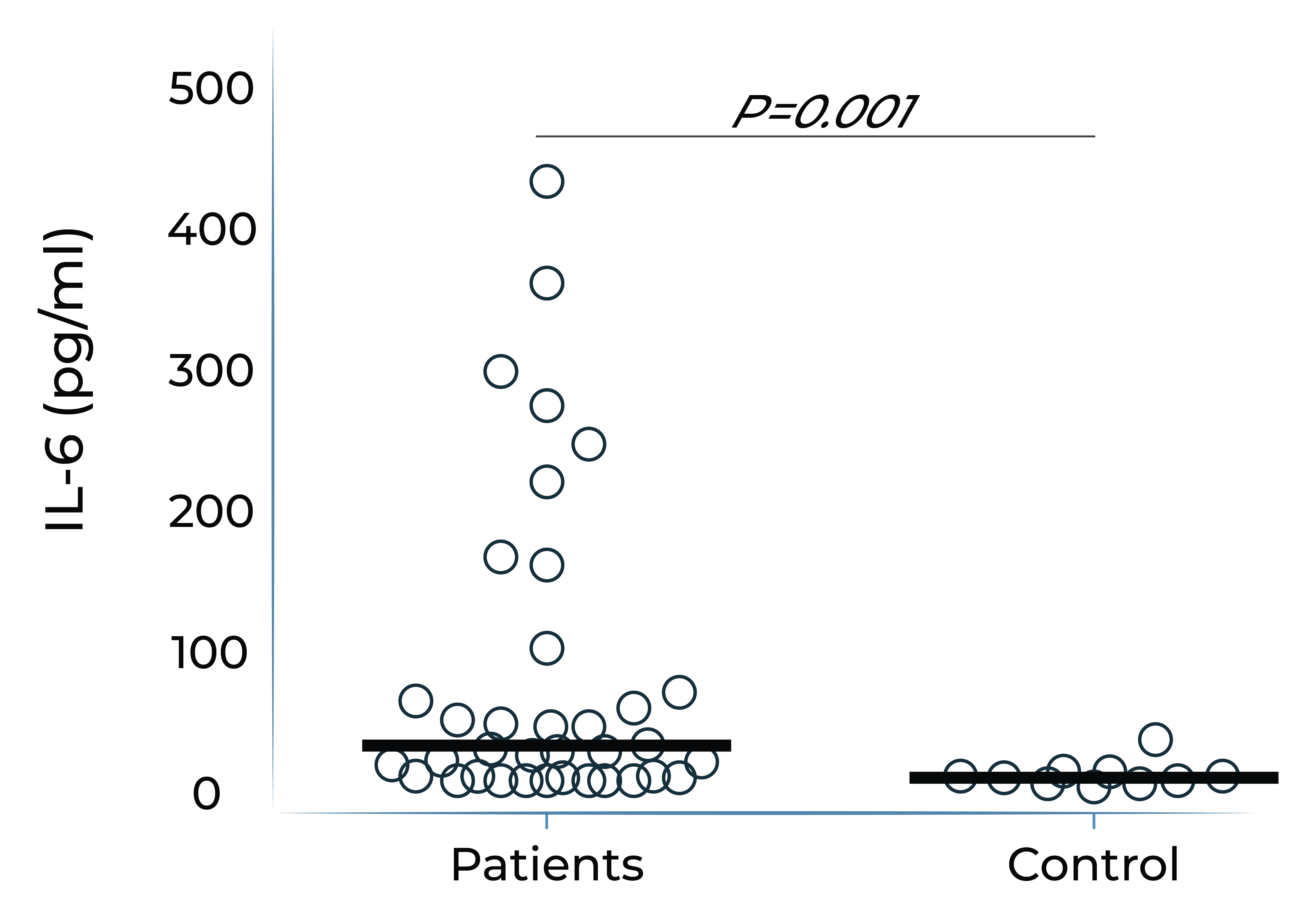
IL-6 levels are significantly elevated in eyes with retinal vascular disease and are implicated in anti-VEGF treatment resistance
Vitreous IL-6 levels in patients with retinal vascular disease vs. control1
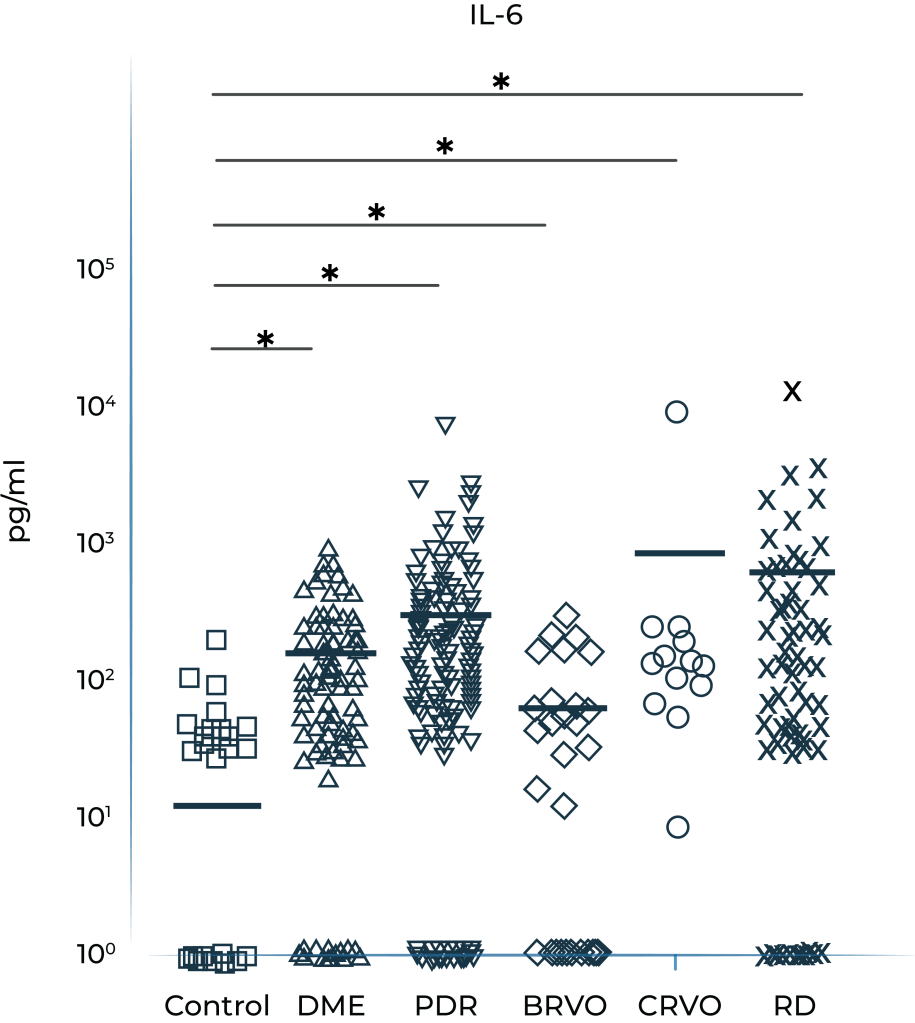
DME: diabetic macular edema; PDR: prolifera tive diabetic retinopathy; BRVO: branch retinal vein occlusion; CRVO: central retinal vein occlusion; RD: retinal detachment.
1.Yoshimura et al. (2009). PLoS ONE 4(12): e8158.
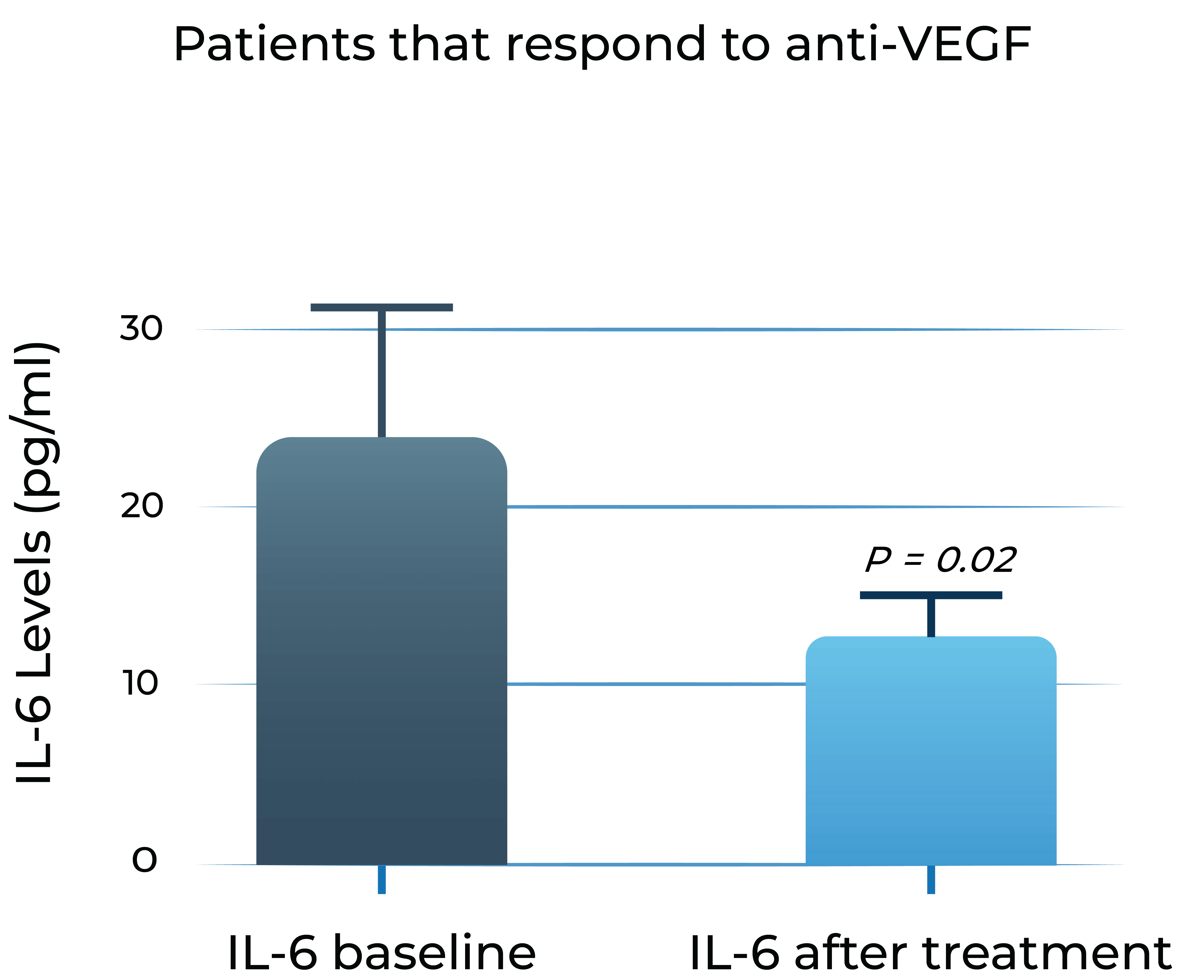
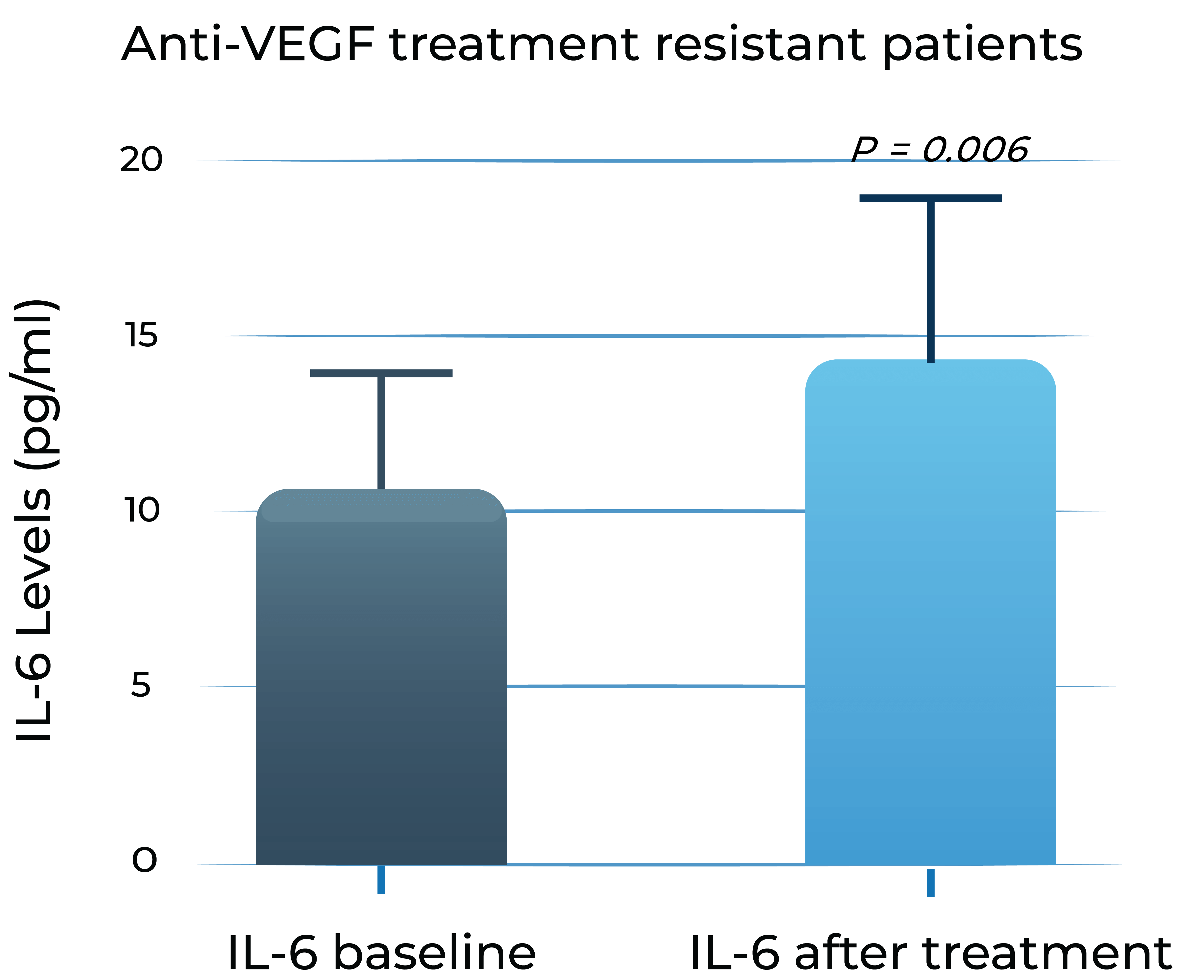
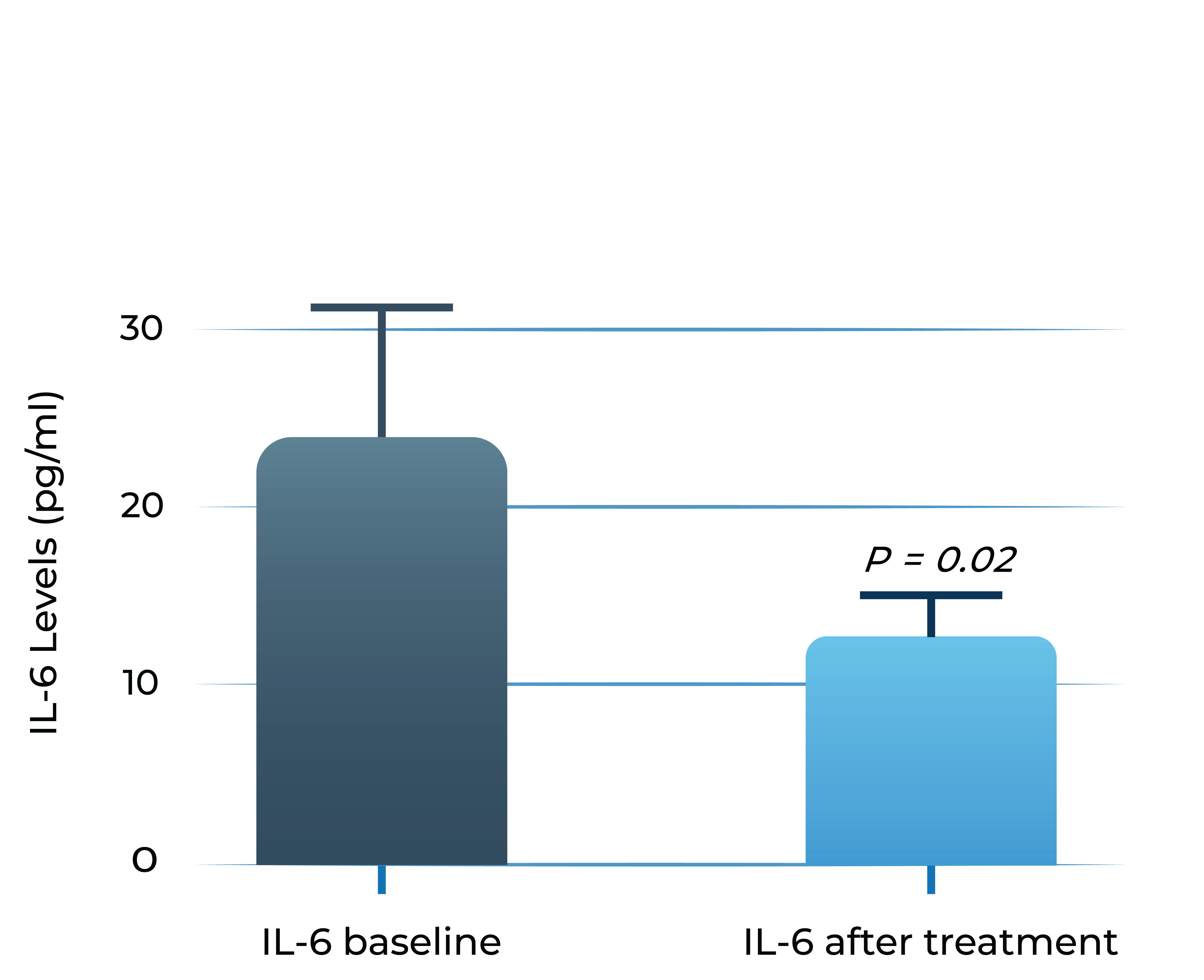
Aqueous humor IL-6 levels significantly correlate with anti-VEGF treatment response in wet AMD2
Patients that respond to anti-VEGF
Anti-VEGF treatment resistant patients
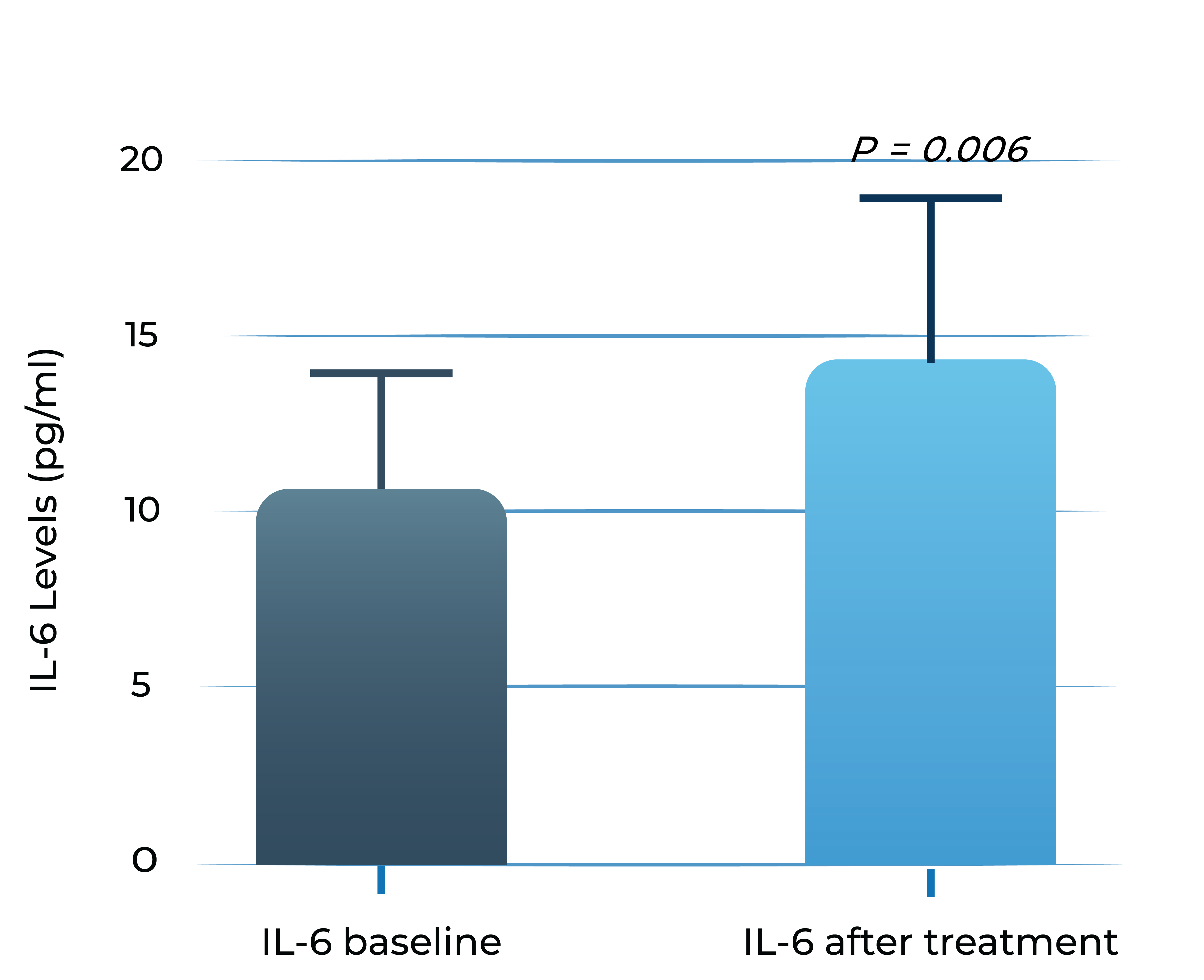
- Adapted from Chalam et al. (2014). Journal of Ophthalmology, Article ID 502174. Mean with SEM plotted.
A significant portion of DME patients (30%-66%) have evidence of persistent disease activity despite frequent anti-VEGF treatment
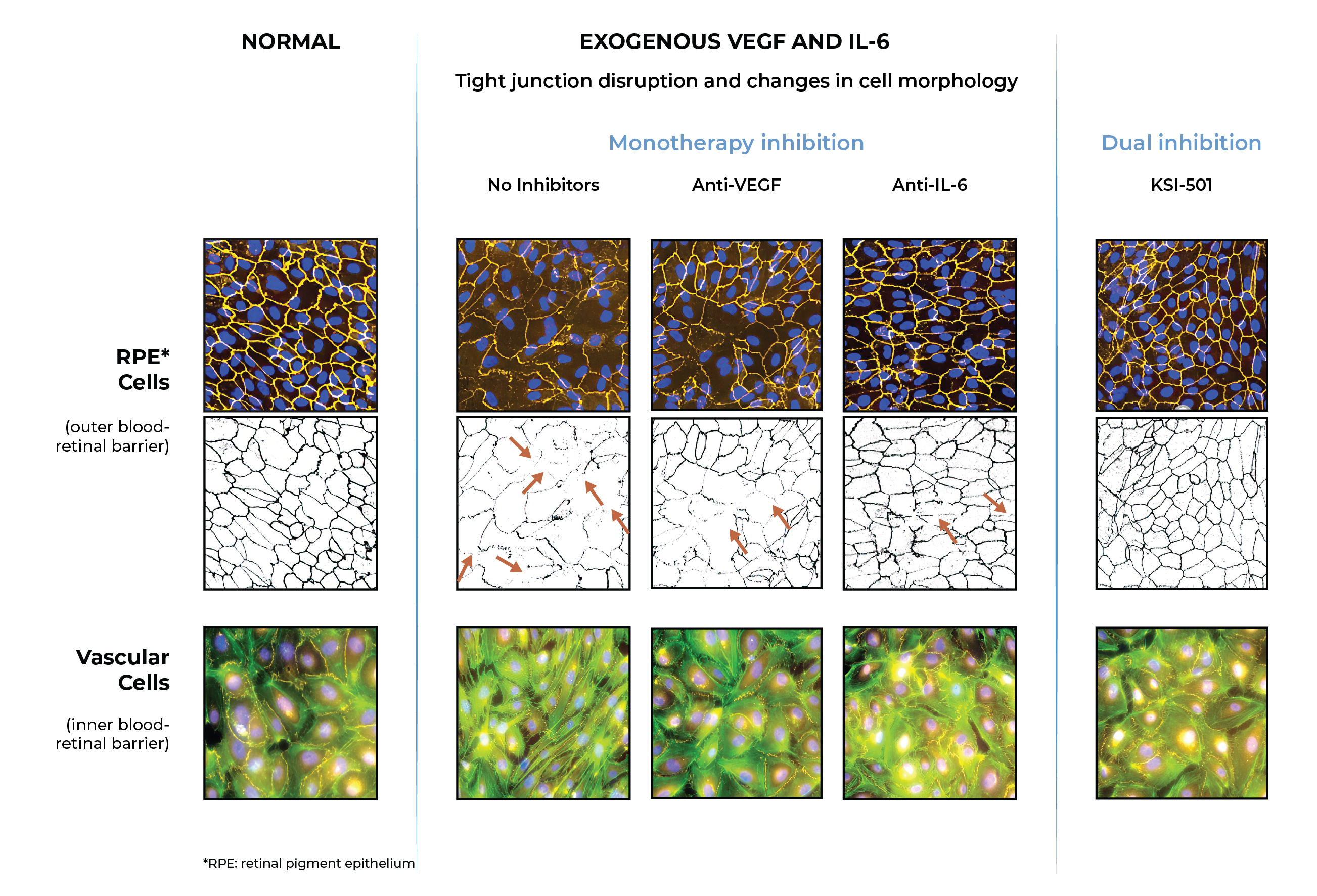
In pre-clinical studies, dual inhibition of VEGF and IL-6 by KSI-501 demonstrated normalization of inner and outer blood retinal barriers compared to anti-VEGF or anti-IL-6 monotherapy
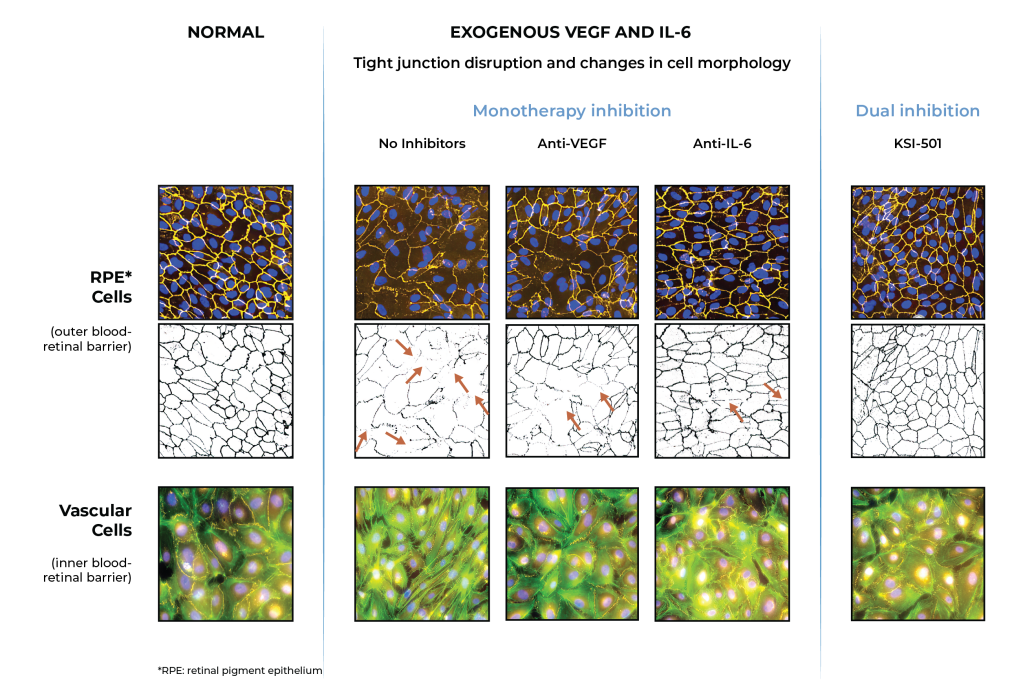
Inner blood-retinal barrier: leakage from vascular endothelium disruption leads to macular edema and hemorrhage
Outer blood-retinal barrier: RPE integrity prevents choroidal vascularization from invading the retina
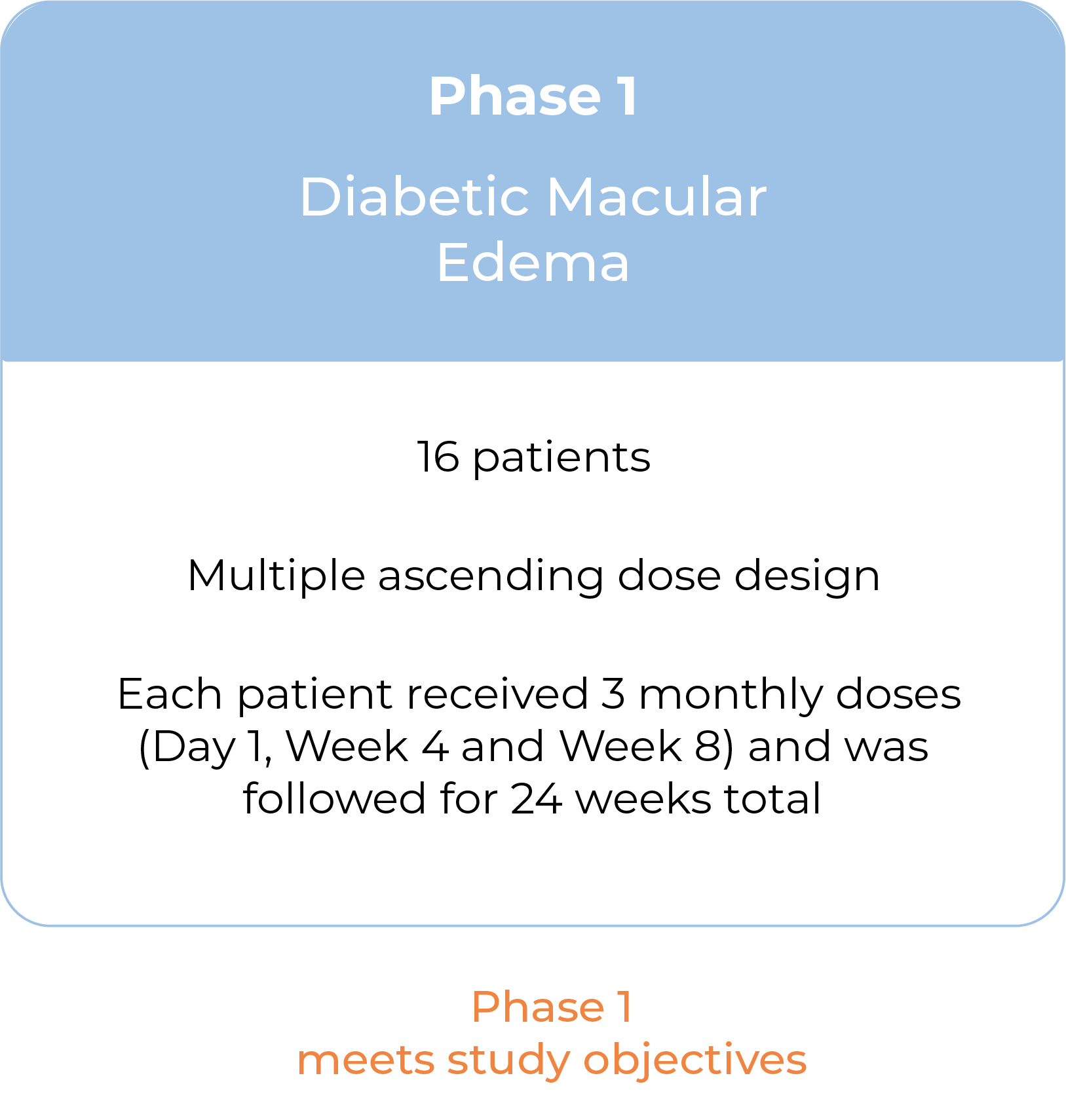
Following Phase 1 results, we plan to advance KSI-501 into a Phase 3 study in wet AMD
Our Phase 1 study in DME, a disease known to have high levels of cytokine-mediated microvascular inflammation in addition to VEGF-mediated vascular permeability, is now complete.
We believe the Phase 1 study results support further clinical development of the KSI-501 program in high-prevalence retinal vascular diseases, such as wet AMD.
In wet AMD, there is preclinical evidence IL-6 is implicated in the development of choroidal neovascularization and clinical evidence demonstrating that IL-6 is associated with development and progression of AMD, resistance to anti-VEGF treatment in wet AMD, and reactivation of disease by promoting growth of new neovascular membranes.
We intend to advance KSI-501 into the Phase 3 DAYBREAK study in 2024 to evaluate its efficacy, durability and safety in wet AMD.

KSI-501 contains three tiers of innovation
Two-target
mechanism of action
![]()
Designed to potently inhibit the IL-6 inflammation pathway and the dominant VEGF pathway
Design based on
our ABC Platform
![]()
Holds the potential for 6-month durability for the majority of patients
Enhanced
formulation
![]()
Informed from tarcocimab’s commercial manufacturing scale up: 50 mg/ml formulation strength
First-in-class
An investigational biologic that addresses the multifactorial nature of macular edema associated with inflammation
In patients with intraocular inflammation, significant vision loss is most commonly a consequence of macular edema. Studies show that inflammation and vascular permeability have a synergistic effect on driving disease progression and vision loss due to macular edema, but there are no approved therapies that target both drivers of disease.
KSI-101 is an investigational, high formulation strength (100 mg/ml) bispecific protein designed to directly target both IL-6-mediated inflammation and edema, and VEGF-mediated vascular permeability.
We intend to develop KSI-101 for patients who have macular edema and inflammation. Currently there are no available intravitreal biologic therapies addressing the spectrum of inflammatory conditions of the retina. Our goal is for KSI-101 to target both underlying disease mechanisms concurrently to prevent vision loss
Patients with vision-threatening retinal inflammatory disease have limited treatment options today
Uveitis is a heterogenous group of diseases characterized by intraocular inflammation. Macular edema is the leading cause of vision loss among uveitis patients. Many patients with macular edema have persistent disease activity despite treatment and are at risk for vision loss.
In macular edema associated uveitis there is no standard treatment algorithm and patients are exposed to therapies with limited efficacy and undesirable side effects.
.png)
.png)
.png)
.png)
- Approximately 30-40% of patients do not respond
- Associated with undesirable ocular and systemic side effects, such as cataract progression and elevated intraocular pressure or glaucoma
- Used as off-label, steroid-sparing agents
- Up to 50% of patients do not have their macular edema resolved
- Approximately 35% of patients do not experience improvement in macular edema
- Adalimumab (anti-TNFa) is currently the only FDA-approved non-steroid therapy for non-infectious uveitis
- Used as a steroid-sparing therapy
- Approximately 55% of patients experienced treatment failure over 85 weeks
- Associated with serious systemic side effects
- Used for patients with persistent macular edema associated with inflammation that fail conventional therapies
- However, the underlying inflammatory component of the pathophysiological process is not addressed by inhibiting VEGF alone
Studies show that both IL-6 and VEGF play a key role in retinal inflammatory disease
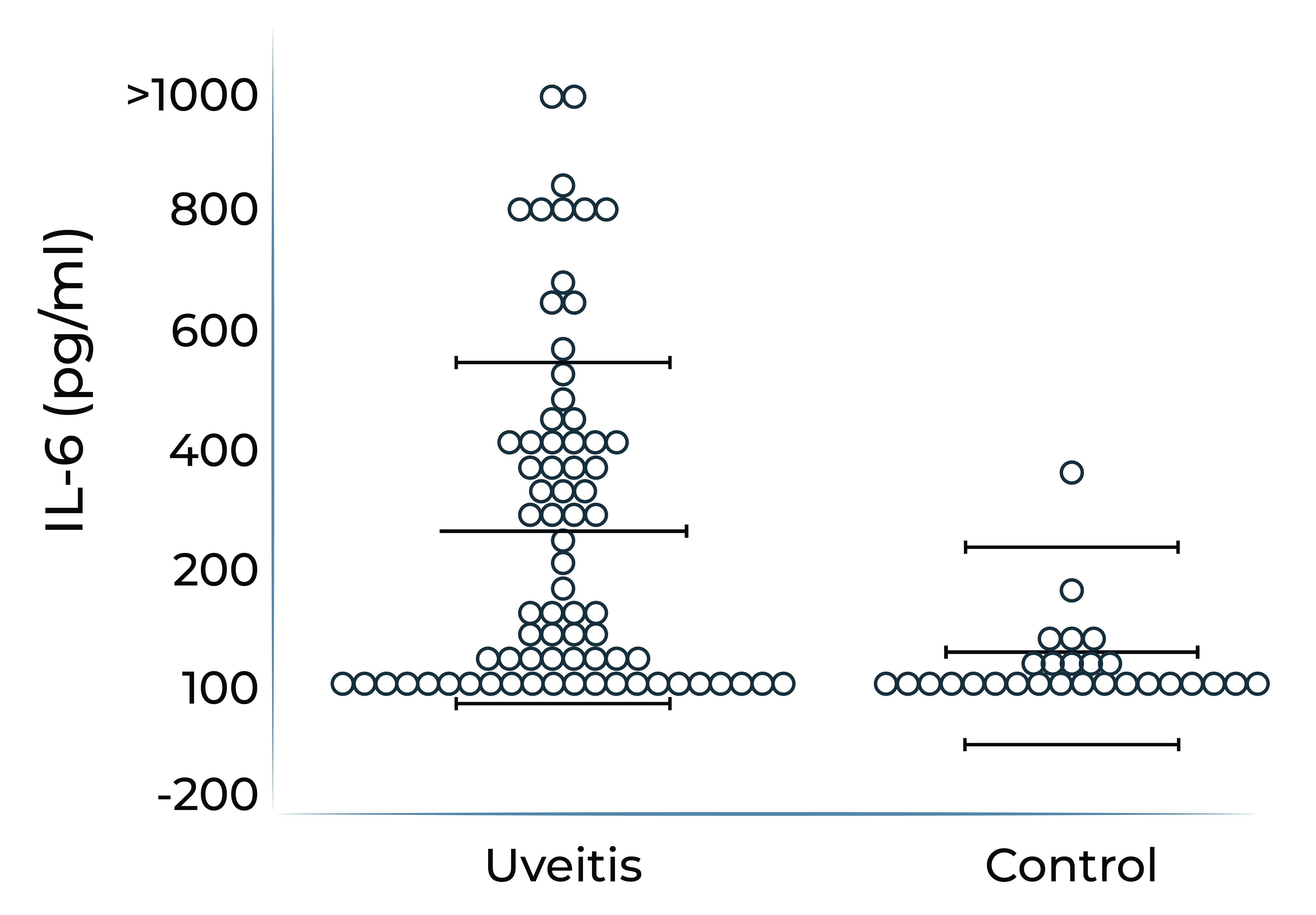
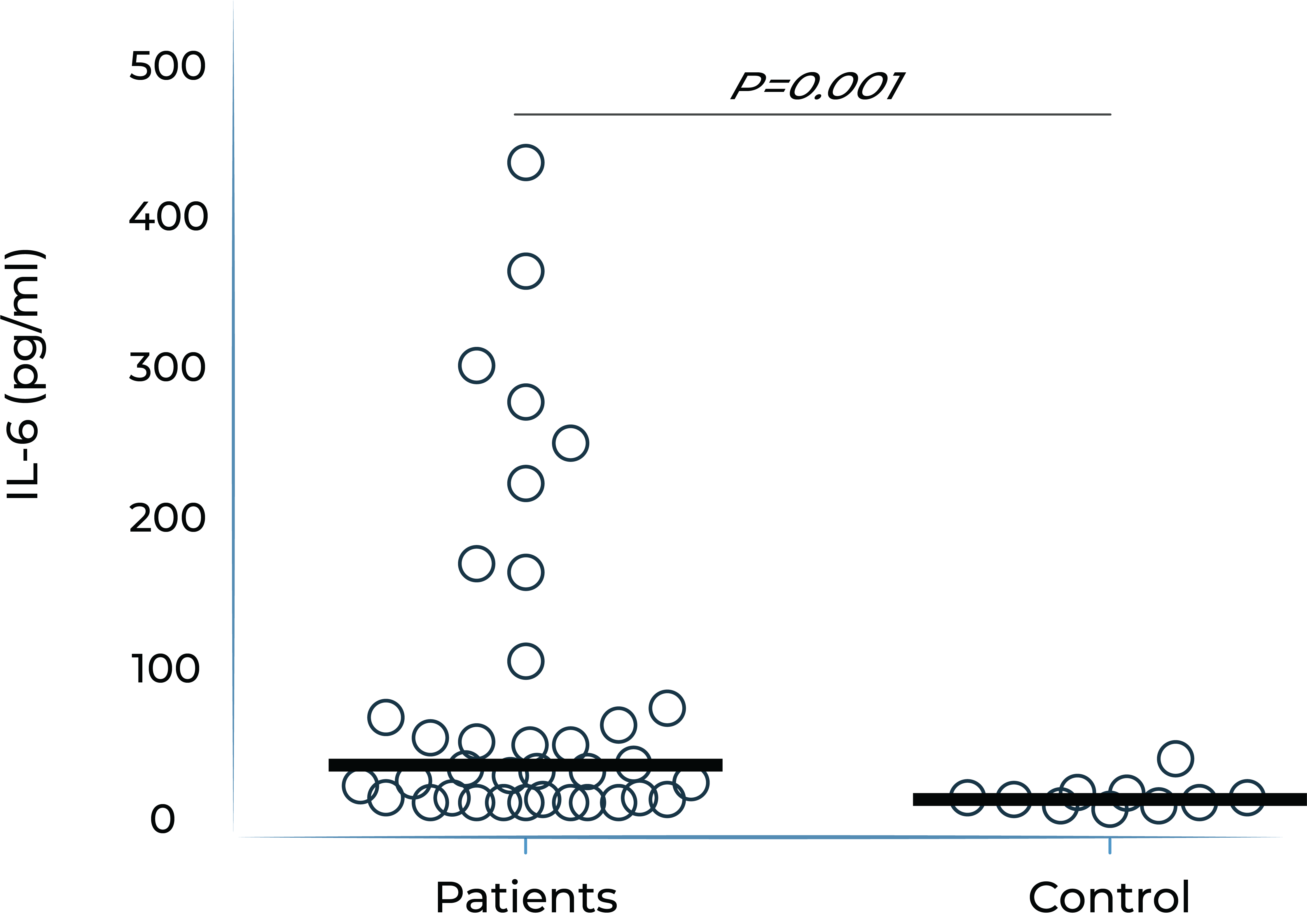
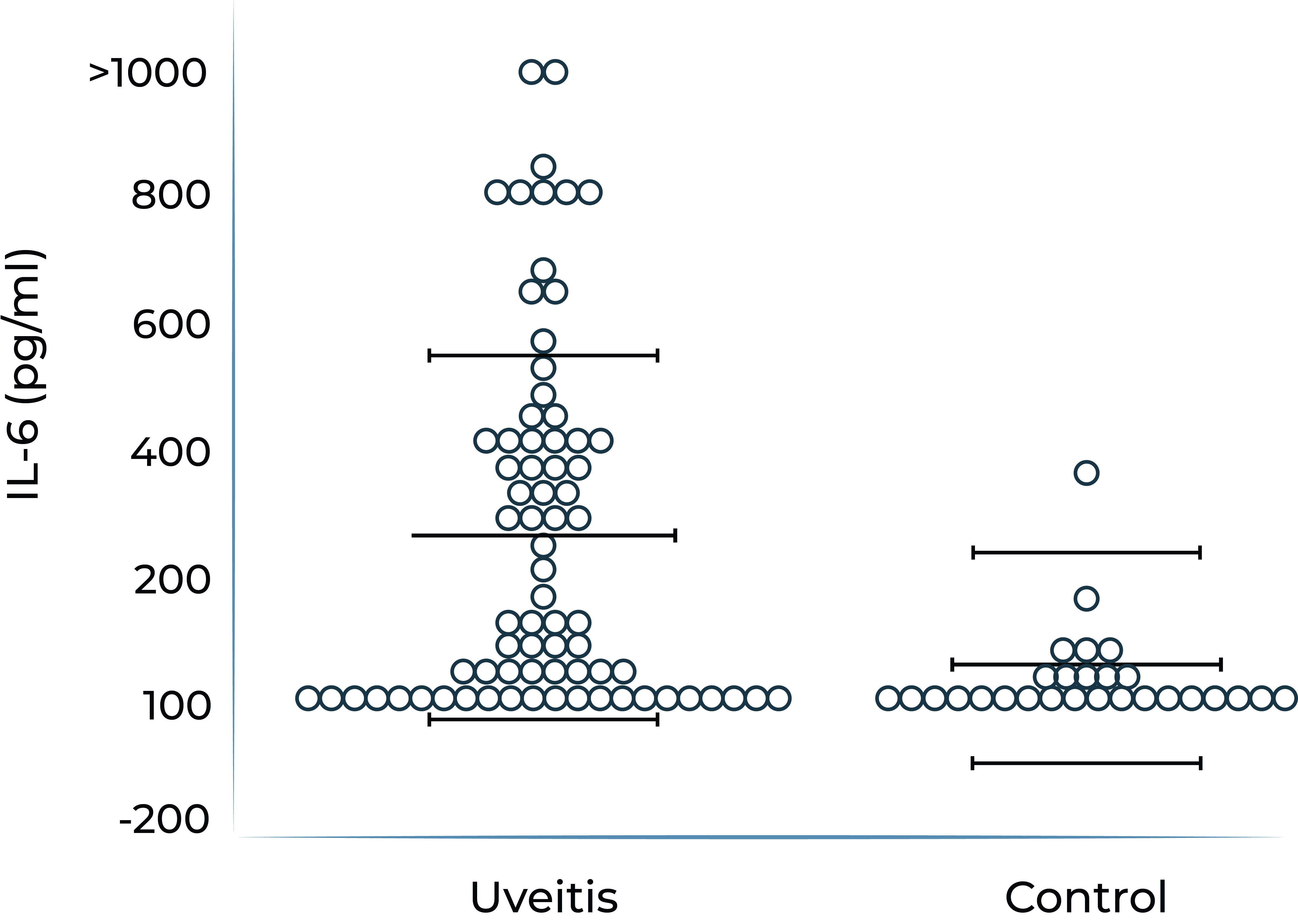
It has been demonstrated that IL-6 levels are elevated in ocular compartments and in serum in patients with non-infectious uveitis, and further elevated in uveitis patients who have macular edema.
Aqueous humor IL-6 levels were elevated in patients with intermediate uveitis1
IL-6 levels are elevated in vitreous fluid of patients with active uveitis2
1. Valentincic et al. Molecular Vision 2011; 17: 2003-2010
2. de Boer et al. Curr Eye Res. 1992;11 Suppl:181-186
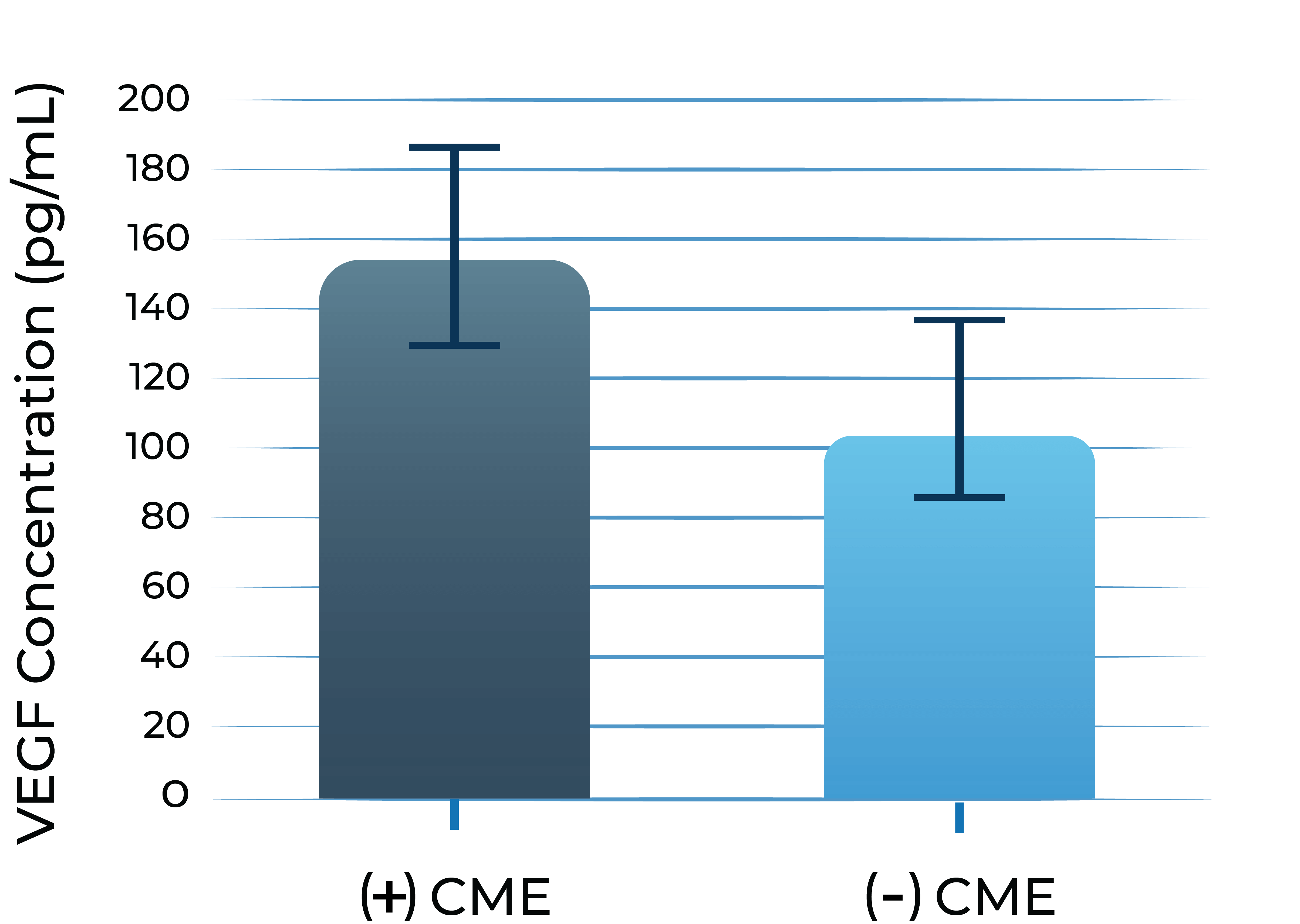
In addition, persistent inflammation triggers VEGF upregulation. Consequently, VEGF levels are found to be elevated in aqueous humor of eyes with uveitis and UME, which can lead to angiogenesis, vascular leakage, and blood-retinal barrier dysfunction.
VEGF levels of patients with uveitis with macular edema vs. without3
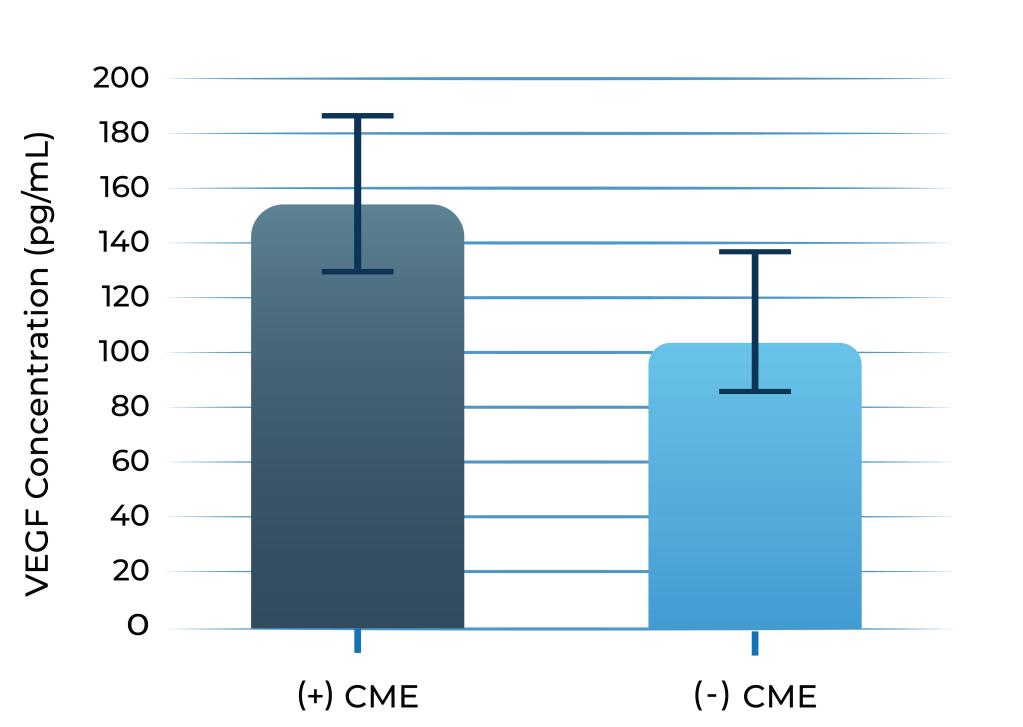
3. Fine et al. Am J Ophthal. 2001; 132: 794-796
KSI-101 is a first-in-class, high formulation strength (100 mg/ml) protein that is well positioned to address the uveitic complex of diseases with macular edema and inflammation for which no available intravitreal biologic therapies exist today
KSI-101 focuses on a market opportunity outside the established anti-VEGF class
We plan to initiate a small dose-finding Phase 1b study of KSI-101 in 2Q 2024 to evaluate its safety and tolerability and to identify two dose levels to progress into pivotal studies. We hope to initiate dual pivotal studies with KSI-101 in 2024.
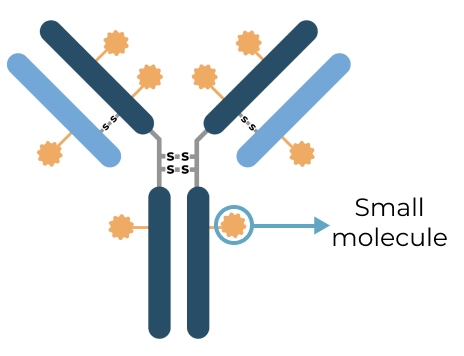
Multi-mechanism, multi-modality targeted biologic for complex retinal and systemic diseases
Triplet medicines combine the benefits of our Antibody Biopolymer Conjugate (ABC) Platform for long-interval dosing of biologics with a new feature that adds sustained release of 100s of small molecules to target three or more mechanisms of action, enabling treatment of complex, multifactorial diseases.
Designing a new generation of targeted therapy for high-prevalence multifactorial diseases

Our triplet ABC medicines aim to broaden what’s possible with antibody conjugate therapies
Antibody Drug Conjugate (ADC) therapies are revolutionizing the way cancer is treated today by delivering highly potent cancer-killing agents directly to cancer cells via a targeted antibody. With our ABC triplet medicines, we aim to build on this foundation in notable ways:
ADC
|
ABC Triplet Medicine
|
Our goal with our triplet medicines is to deliver greater therapeutic benefit for multifactorial diseases in the eye and systemically by modulating multiple distinct pathological processes in parallel

.png)

.png)
.png)